Modification and application of nitrile hydratase amino acid motif
A nitrile hydratase and amino acid technology, applied in the field of bioengineering, can solve the problems of narrow substrate spectrum and low catalytic efficiency, and achieve the effect of improved catalytic performance and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Construction of each mutant of PTNHase
[0034] The wild-type plasmid pET24a Pt NHase of (+) - Pt WT (. This plasmid is described in Cheng et al, Computational Design of Nitrile Hydratase from PseudonocardiathermophilaJCM3095 for Improved Thermostability, 2020), all-plasmid PCR was constructed Mutant Plasmid pET24a (+) - M46K, L6T, F126Y and its combined mutants M46K-L6T, M46K-F26Y and M46K-L6T-F126Y. First, the plasmid PET24a (+) - Pt WT is a template, the mutant sequence is designed on the primer, and the DNA fragment of the base sequence is amplified by PCR, and the primer sequence used is shown in Table 1, and the amplification system is shown in Table 2. Accordingly, the PCR amplification reaction conditions were 95 ° C for 3 min, 98 ° C degeneration 15 s, 55 ° C for 1 min 45 s, 72 ° C for 5 min, and a total of 30 cycles. The PCR product was digested for 2-3 h with DPNI digestive enzyme, converting E.Coli DH5α, after the culturing of LB plate, picked the sin...
Embodiment 2
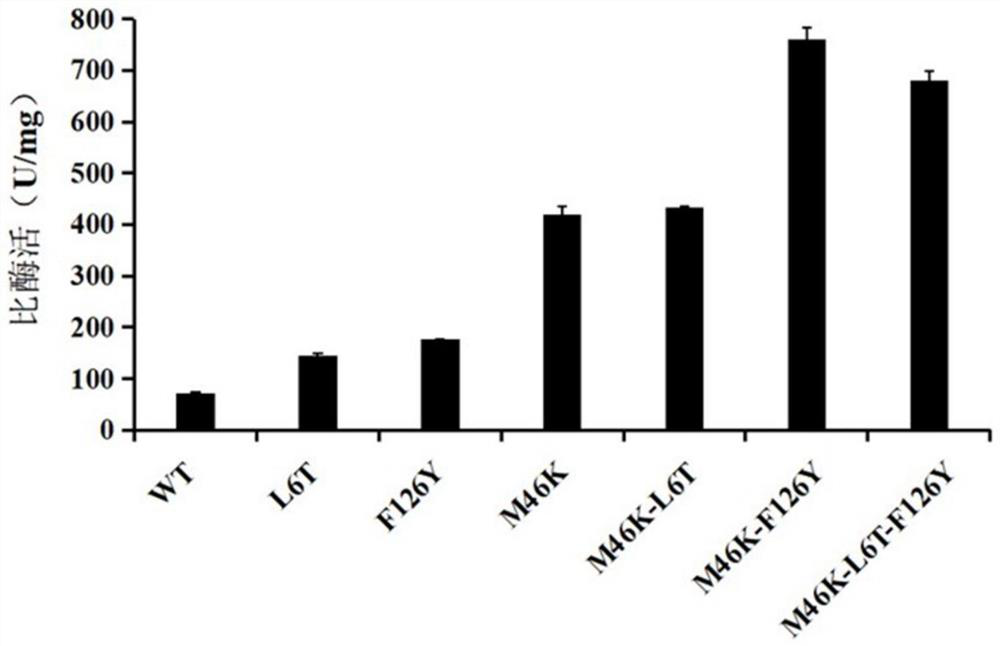
[0040] Example 2: Catalytic Efficiency of Wild Type and Mutant of Pt Nhase
[0041] PT Nhase's wild-type WT and recombuquis PET24A (+) - M46K, PET24A (+) - L6T, PET24A (+) - F126Y and combined mutant PET24A (+) - M46K-L6T, PET24A (+) - M46K-F26Y and PET24A (+) - M46k-L6T-F126Y were converted to E.Coli BL21 (DE3), and were colored to 5 ml of Lb medium, 37 ° C, 200 rpm. The seed fluid was transferred to 1% (V / V) to 100 mL 2 × YT medium, 37 ° C, 200 rpm, and cultured to OD600 to 0.6-0.8, and the final concentration of 0.4 mm isopropyl phosphidide (Iptg) and 0.1g / L CoCl 2 · 6h 2 O, the changed culture temperature was 24 ° C, induced expression of 12-16h.
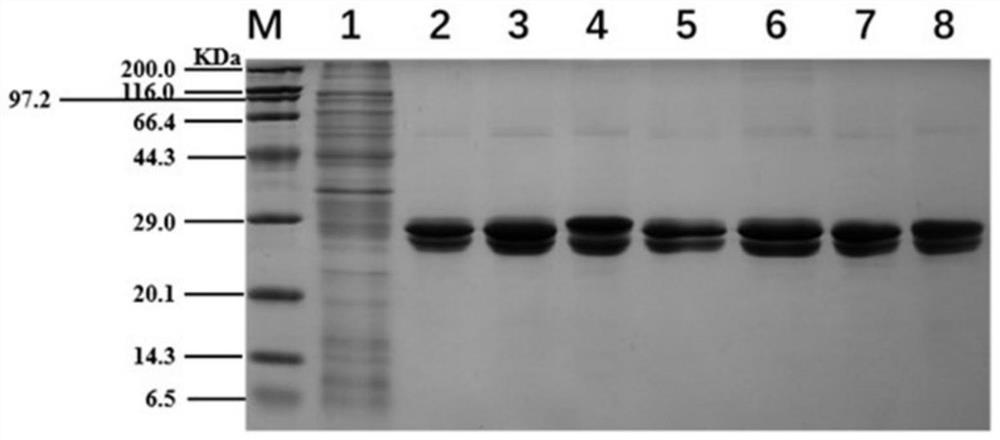
[0042] Purification of wild-type WT and its six mutants, using SDS-PAGE detection of protein purification quality, detection figure 1 As shown, the protein expressed by wild type and its mutants can be seen in the purified protein strip, and the purification quality is high.
[0043] The concentration of WT and its mutant enzyme...
Embodiment 3
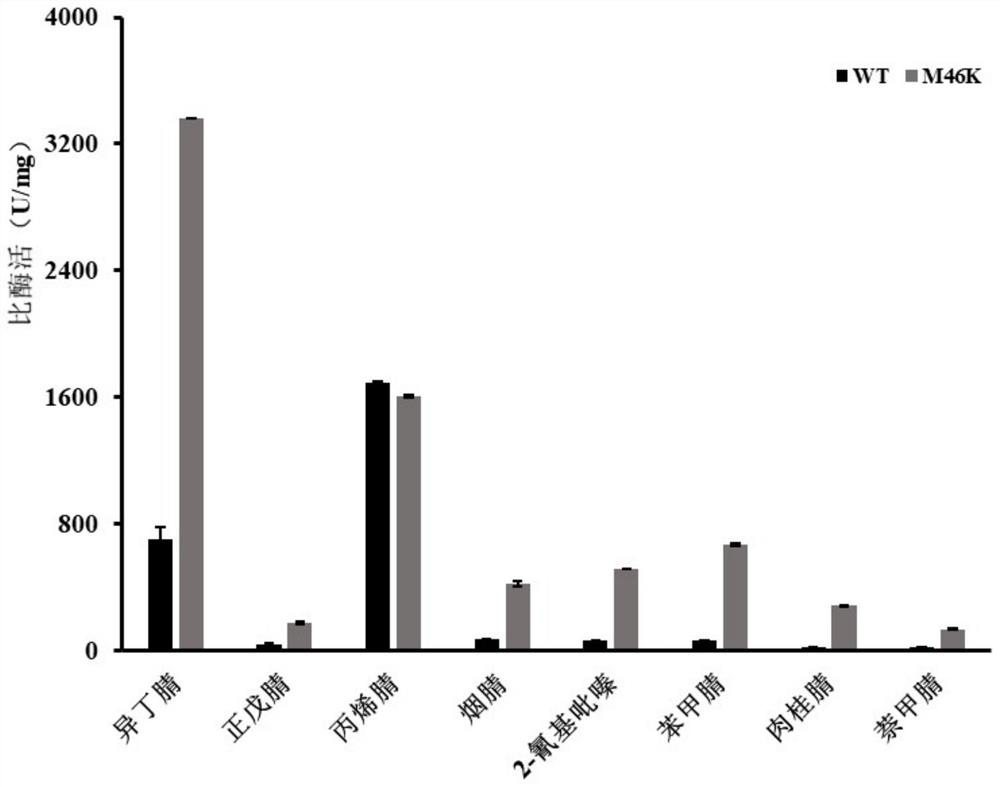
[0047] Example 3: Pt Nhase's wild-type and mutant M46K substrate
[0048] Culture induction and reaction system as in Example 2. Based on the above-mentioned pure enzymes, different substrate catalytic reactions were carried out, of which isobutyronitrile, acrylonitrile, caminon nitrile and benzonitrile catalytic reaction had a concentration of 0.05 mg / ml, pentarnile, nitrile and 2-cyanopyrazine. The concentration of the reaction was 0.5 mg / ml, the concentration of naphthalene catalyzed reaction was 0.01 mg / ml.
[0049] Liquid phase detection method: The flow phase is a acetonitrile: water = 1: 2 (v / v); flow rate: 1 ml / min, isobutyl amide, pentamide, acrylamide, nicotinamide, The pyrazinamide and naphthyl amide are 0.6 ml / min; detection wavelength: isobutamide and pentamide are 202 nm, acrylamide, nicotinamide, benzamide and naphthoamide are 215 nm, pyrazinamide and cinnamide 261 nm The column temperature was 40 ° C, measuring the amount of product amide in the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com