Method for simultaneously detecting or identifying effective components in heart-soothing lipid-lowering tablet
An active ingredient and lipid-lowering technology, which is applied in the field of detection or identification of active ingredients in Shuxin Jiangzhi Tablets and simultaneous detection or identification of active ingredients in Shuxin Jiangzhi Tablets. TLC, mutual interference and other problems of polydatin and polydatin, to achieve the effect of saving detection time and cost, strong specificity, and improved specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
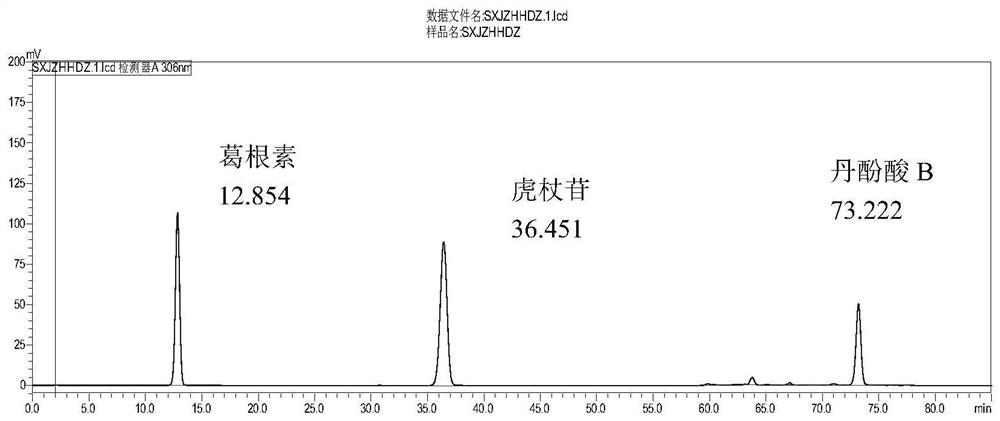
[0045] Embodiment 1HPLC measures the content of puerarin, polydatin, salvianolic acid B
[0046] The present embodiment simultaneously detects the method for active ingredient in Shuxin Jiangzhi Tablets, as follows:
[0047]1. Preparation of reference substance solution: Take appropriate amount of puerarin, polydatin and salvianolic acid B reference substance, weigh them accurately, add dilute ethanol to make puerarin containing 190 μg per 1 ml, polydatin per 1 ml containing 75 μg, salvianolic acid Each 1ml of B contains 240μg mixed control solution.
[0048] 2. Preparation of the test solution: Accurately weigh 0.3g of Shuxin Jiangzhi Tablets, put it in a 10ml measuring bottle, add dilute ethanol to the mark, shake well, ultrasonicate, power 250W, frequency 50kHz, process 20min, use 0.45μm micrometer Pore membrane filtration, that is.
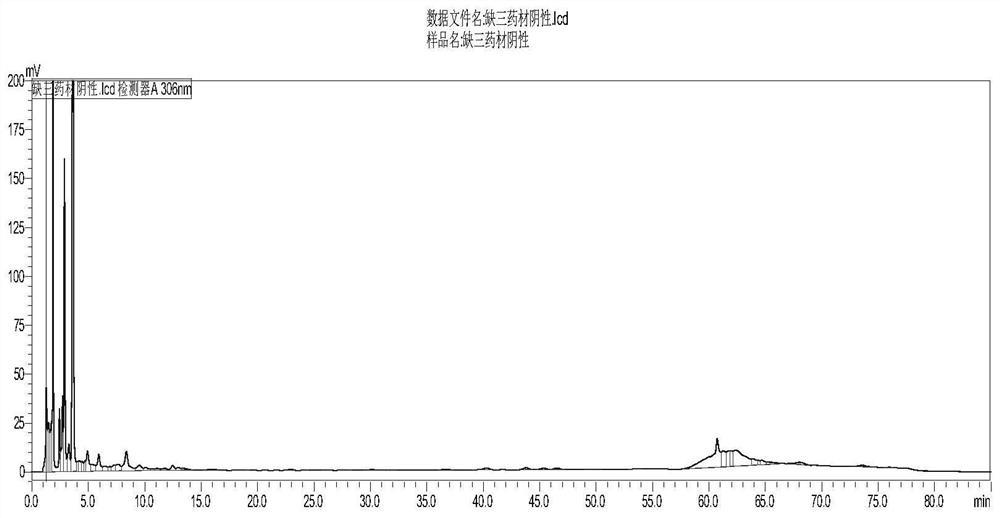
[0049] 3. Preparation of negative control solution: Accurately weigh 0.3g of the negative sample lacking puerarin, polydatin, and salvian...
Embodiment 2
[0079] The content of puerarin, polydatin, salvianolic acid B in the embodiment 2 HPLC determination Shuxin Jiangzhi Tablets
[0080] The present embodiment simultaneously detects the method for active ingredient in Shuxin Jiangzhi Tablets, as follows:
[0081] 1. The preparation of reference substance solution, the preparation of negative control solution: with embodiment 1.
[0082] 2. Preparation of the test solution: take the test samples SXJZ-20190301, SXJZ-20190302, SXJZ-20190303 and the marketed products 20161102, 20170404, 20170701, the preparation method is the same
[0083] Example 1.
[0084] 3. Chromatographic conditions and system suitability test: the same as in Example 1.
[0085] 4. Determination method: respectively draw and mix 10 μL of the reference substance solution and the test solution, inject it into the liquid chromatograph, measure it, and obtain it.
[0086] 5. Results: All components met the baseline analysis requirements. The theoretical plate n...
Embodiment 3
[0089] Example 3 Identification of the same puerarin and polydatin TLC
[0090] In this embodiment, 3 batches of products (batch numbers: 20161102, 20170404, 20170701) and 1 batch of laboratory samples are identified, as follows:
[0091] Take Shuxin Jiangzhi Tablets, grind finely, accurately weigh 5g, add 20ml of methanol, ultrasonicate for 20 minutes, filter, and concentrate the filtrate to 10ml as the test solution.
[0092] Take 3g of the negative sample lacking kudzu root and Polygonum cuspidatum, grind it finely, add 20ml of methanol, ultrasonicate for 20 minutes, filter, concentrate the filtrate to 10ml, and use it as the negative sample solution.
[0093] Another puerarin, polydatin reference substance, were added methanol to make each 1ml solution containing 1mg, as the reference solution.
[0094] According to the thin-layer chromatography test, absorb 5 μl of each of the above four solutions, and spot them on the same high-efficiency silica gel G thin-layer plate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com