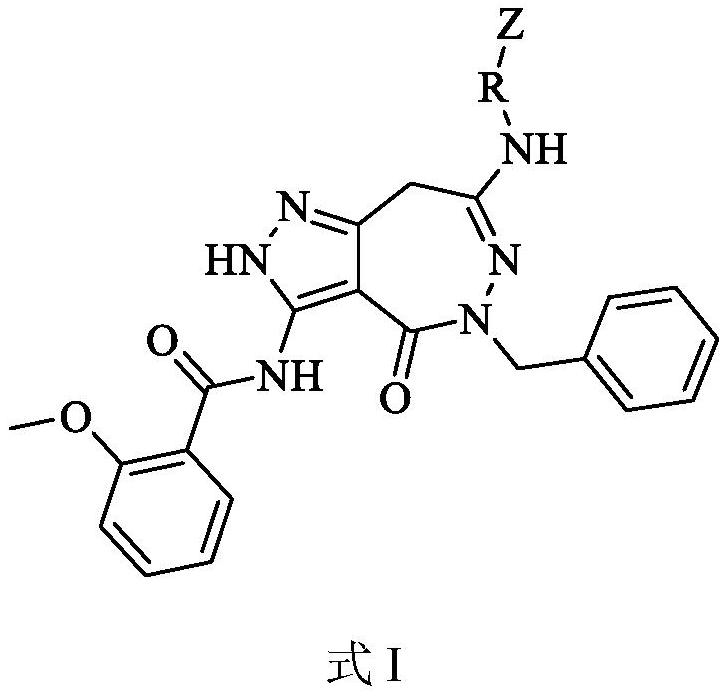
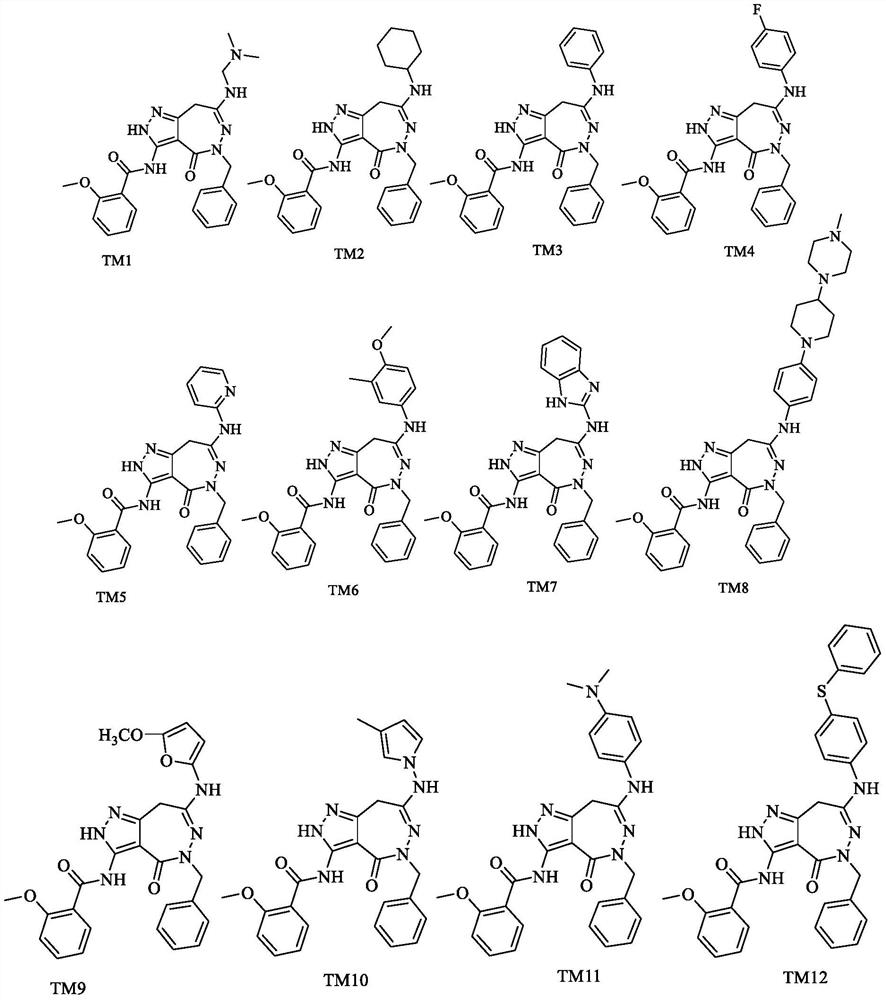
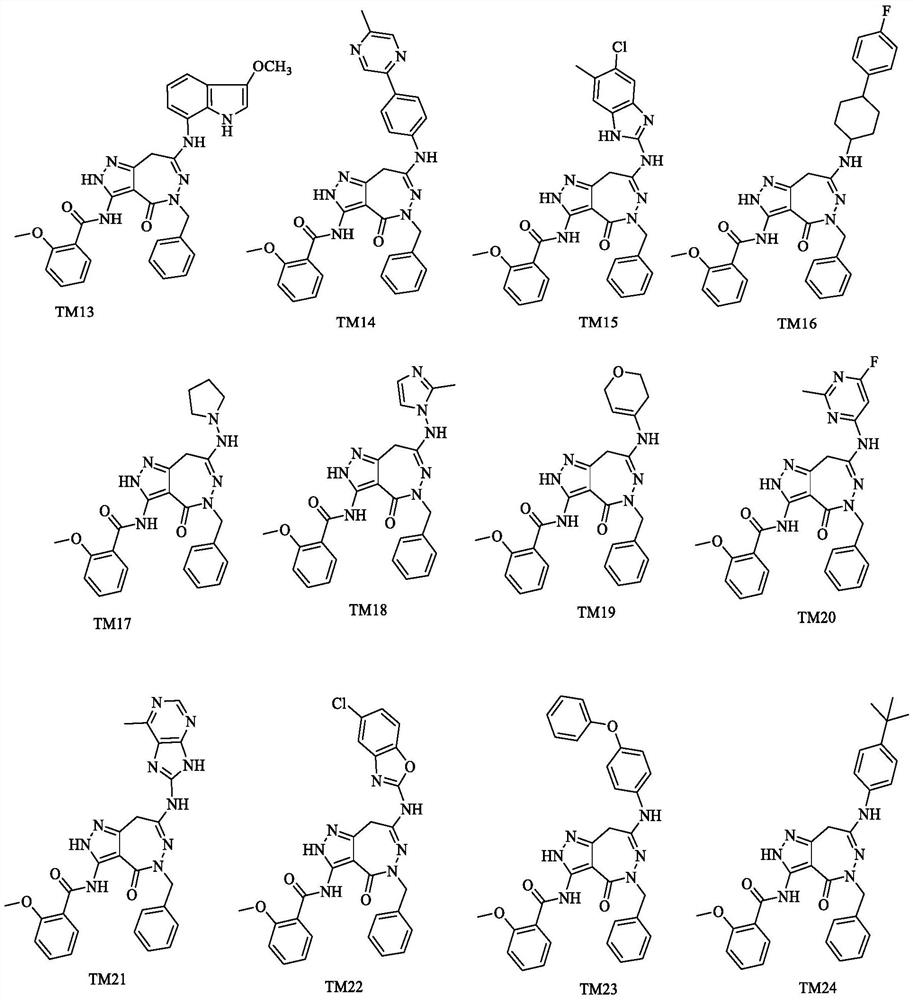
Pyrazolo-oxo-diaza compound as BTK inhibitor
A compound and pyrazolo technology are applied in the field of pyrazolo oxodiazepine compounds and their preparation, and can solve the problems of affecting drug efficacy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Preparation of Compound 2
[0115]
[0116] The ethanol (1500 g) was added to ethanol (3000 mL), stirred at room temperature for 1 h, the reaction mixture was added to propennitrile (1280 g), the temperature control reflow reaction, the reaction was completed, the reaction liquid was lowered to 20 ° C, stirred into crystallization 2h, crystallization After the filtration, the filter cake was dried with 0 ~ 5 ° C ethanol (400 mL), dried at 35 ° C; dried to three bottles, poured into purified water (5000 mL), stirred at room temperature to dissolve, 5 mol / L hydrochloric acid is adjacent to 3 to 4, there is a white solid precipitation, after the washing is complete, filter, filter cake with purified water (500ml), whose white needle crystal, 35 ° C drying to constant weight, Intermediate compound 2, yield 82%, purity 97.2%.
Embodiment 2
[0118] Preparation of Compound 3
[0119]
[0120] Compound 2 (200 g) was added to purified water (500 mL), after stirring, hydrazine (50 mL) was added while stirring, and the reaction temperature reflux reaction was added, the reaction was completed, the reaction liquid was lowered to 0 ° C, analysis. After the crystal is completed, the filter cake is dried down to the constant weight of 35 ° C to constant weight, and the intermediate compound 3, the yield is 86%, and the purity is 98.1%.
Embodiment 3
[0122] Preparation of Compound 4
[0123]
[0124] NaOH (200 g) was added to purified water (500 mL), stirred, and the compound 3 (40 g) was added, and the temperature was refluxed for 10 h, the reaction was completed; the reaction liquid temperature was 0 to 10 ° C, drip 5 mol / L hydrochloric acid to regulate the solution pH Value to 3 ~ 4, there is a white solid precipitation, all precipitation, filtration, filter cakes 35 ° C to constant weight, and the white solid intermediate compound 4, yield 92%, purity 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com