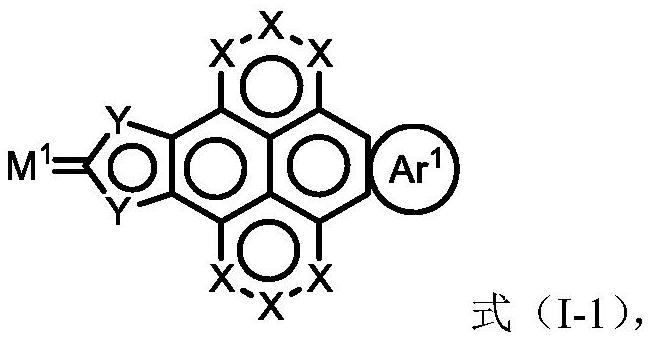
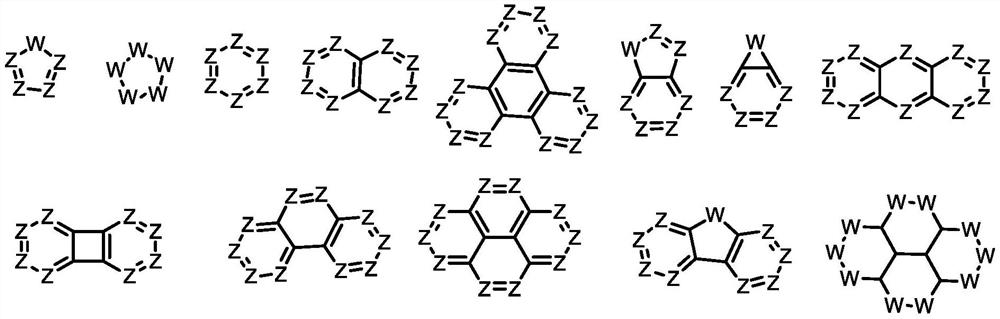
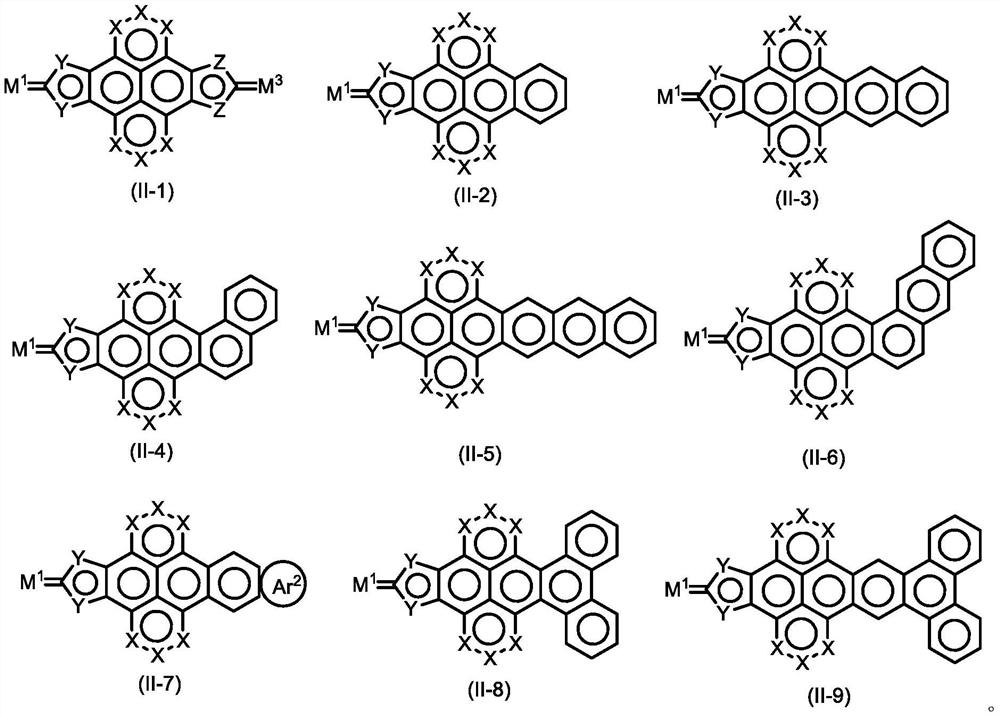
Aromatic ring pyrene quinone compound and application thereof
A compound and aromatic ring technology, applied in the field of organic electroluminescence, can solve problems such as insufficient stability, reduced lifespan, and reduced lifespan of organic light-emitting diodes, achieve excellent hole transport properties and stability, prolong life, and improve electroluminescence. The effect of luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0170] The synthesis method of the compound according to the present invention is exemplified, but the present invention is not limited to the following examples.
[0171] (1) Compound synthesis part
Embodiment 1
[0172] Embodiment 1 synthetic compound DPH-1
[0173]
[0174] Synthesis of compound A2:
[0175] Compound A1 (2.02g, 10mmol), sodium periodate (NaIO 4 17.6g, 81.8mmol), ruthenium trichloride (RuCl.XH 2 (0, 0.25g1.2mmol), acetonitrile 40mL, dichloromethane 40mL, and distilled water 50mL were stirred overnight at 30-40 degrees, the reaction product was cooled to room temperature, and 200mL distilled water was added, then the precipitate formed by filtration under reduced pressure was used Extract the filtrate with dichloromethane, and add anhydrous magnesium sulfate to it to dry and remove moisture, then remove the solvent under reduced pressure to obtain a crude product, then use column chromatography to separate and purify (dichloromethane is the eluent) to obtain the product, and further vacuum The product was dried to prepare the desired solid compound A2 (0.8 g, yield 31%), MS: [M+H] + =263.
[0176] Synthesis of Compound A3:
[0177] Dissolve compound A2 (2.62g, ...
Embodiment 2
[0189] Embodiment 2 synthetic compound DPH-2
[0190]
[0191] Synthesis of compound DPH-2:
[0192] Under nitrogen condition, titanium tetrachloride (4.67g, 25mmol), A9 (3.08g, 20mmol), A7 (4.36g, 10mmol) was refluxed and stirred in nitrogen dry pyridine / dichloromethane for 24 hours, then concentrated with cold Quenched with hydrochloric acid, concentrated in dichloromethane, then dried with anhydrous sodium sulfate, distilled under reduced pressure, and the residue was recrystallized with DCM / MeOH to obtain DPH-2 (2.96g, yield 42%), MS: [M+H ] + =705.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com