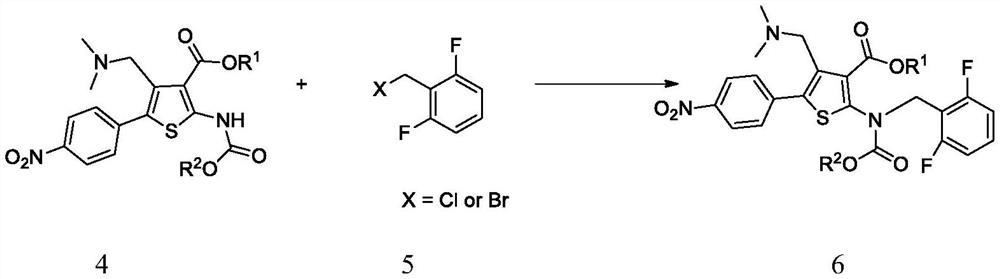
A kind of synthetic method of Relugoli or its salt
A synthesis method and compound technology, applied in the field of medicine and chemical industry, can solve the problems of difficult removal of dimerization impurities, high cost, high cost of enlarged production route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079]
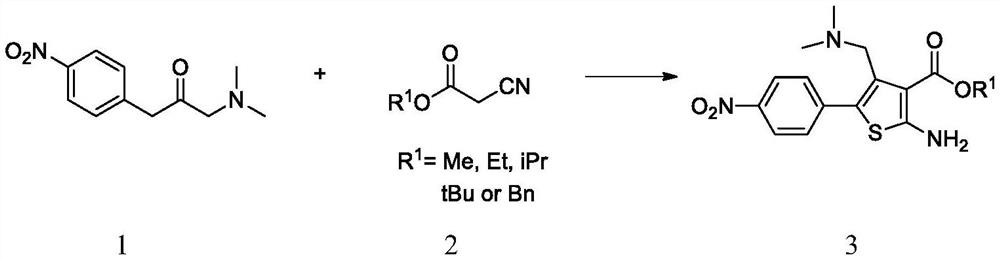
[0080] Compound 1 (22.22g, 100mmol), ethyl 2-cyanoacetoacetate 2a (11.88g, 105mmol) and ethanol (111mL) were added to the three-necked flask, and after stirring and dissolving, diisopropylethylamine (25.85g, 200mmol) was added dropwise. ), added sulfur powder (3.37 g, 105 mmol), stirred evenly, heated to 75~80 ℃ and reacted overnight. After the reaction was completed, dilute hydrochloric acid (3%, 222 mL) was added to quench the reaction, part of the ethanol was removed, ethyl acetate was added to extract and the organic phase was discarded, the aqueous phase was collected, and sodium bicarbonate solution was added to adjust the pH to 8 to 9, and a large amount of solid was precipitated , and slowly cooled and crystallized, filtered and dried to obtain compound 3a (29.18 g, 83.5%).
[0081] MS(ESI)m / z=350.1[M+H] +
[0082] 1 H NMR(500MHz, DMSO)δ8.23(d,J=8.8Hz,2H),7.79(d,J=8.8Hz,2H),7.60(s,2H),4.24(q,J=7.2Hz,2H) ), 3.55(s, 2H), 2.07(s, 6H), 1.32(t, J=7.2Hz, 3H)....
Embodiment 2
[0085]
[0086]Compound 1 (22.22 g, 100 mmol), isopropyl 2-cyanoacetoacetate 2b (13.35 g, 105 mmol) and isopropanol (111 mL) were added to the three-necked flask, and after stirring and dissolving, diisopropylethylamine (25.85 mmol) was added dropwise. g, 200 mmol), added sulfur powder (3.37 g, 105 mmol), stirred evenly, heated to 80~85 ℃ and reacted overnight. At the end of the reaction, dilute hydrochloric acid (3%, 222 mL) was added to quench the reaction, part of the isopropanol was removed, ethyl acetate was added for extraction and the organic phase was discarded, the aqueous phase was collected, and sodium bicarbonate solution was added to adjust the pH to 8 to 9. A large amount of solid was slowly cooled and crystallized, filtered and dried to obtain compound 3b (30.71 g, 84.5%).
[0087] MS(ESI)m / z=364.2[M+H] +
[0088] 1 H NMR(500MHz, DMSO)δ8.24(d,J=8.8Hz,2H),7.80(d,J=8.8Hz,2H),7.58(s,2H),4.74-5.08(m,1H),3.53 (s, 2H), 2.08(s, 6H), 1.18(d, J=6.4Hz, 3H).
[008...
Embodiment 3
[0091]
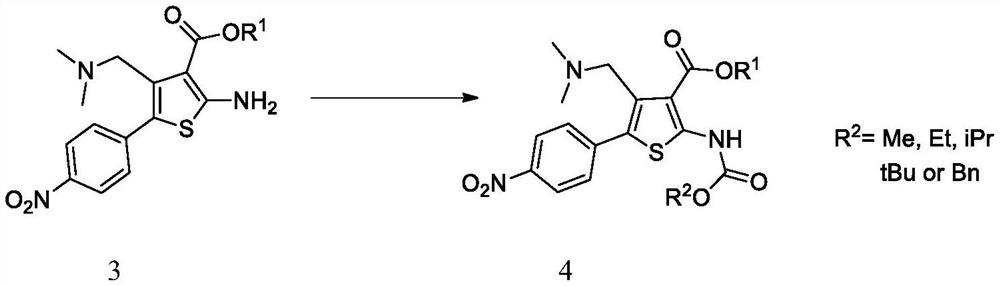
[0092] Compound 3a (34.94 g, 100 mmol) was added to the three-necked flask, 175 mL of dichloromethane was added, and the mixture was stirred and dissolved. The reaction flask was placed in an ice bath and cooled to 0-5° C., triethylamine (20.24 g, 200 mmol) was added, methyl chloroformate (10.39 g, 110 mmol) was slowly added dropwise, and the temperature was raised to room temperature to react overnight. After the reaction was completed, water (349 mL) was added, 175 mL of dichloromethane was added to extract and the aqueous phase was discarded, the organic phase was collected, washed with water, concentrated to a small volume, n-heptane was added, slowly cooled and crystallized, filtered and dried to obtain compound 4a (35.90 g, 88.1%). MS(ESI)m / z=408.1[M+H] + , 1 H NMR (400MHz, CDCl 3 )δ10.55(s,1H),8.26(d,J=8.8Hz,2H),7.72(d,J=8.8Hz,2H),4.30-4.56(m,2H),3.79(s,3H), 3.66(s, 2H), 2.12(s, 6H), 1.33(t, J=7.2Hz, 3H).
[0093] In embodiment 3, methyl chloroformate c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com