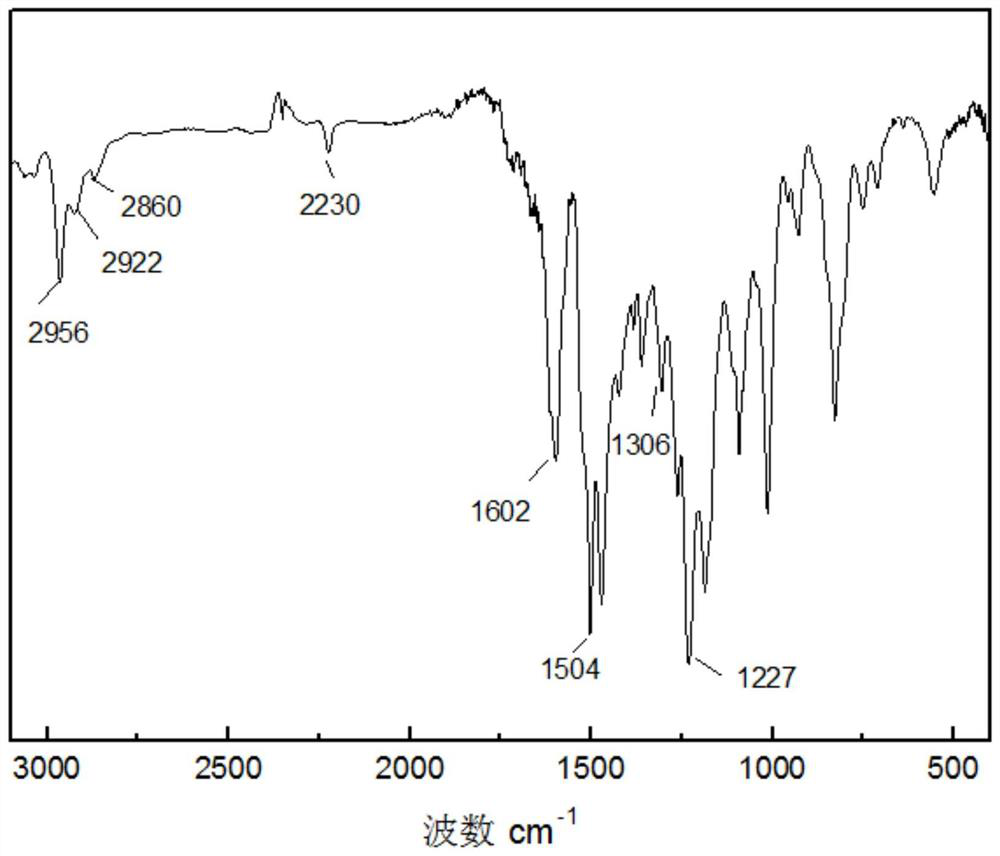
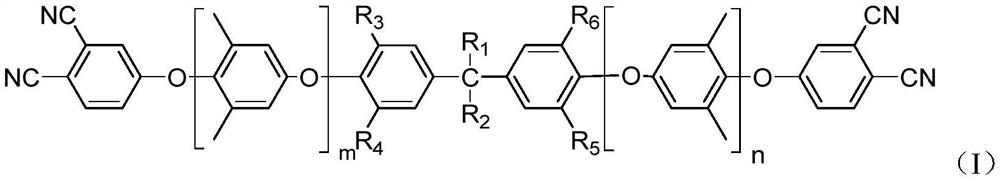
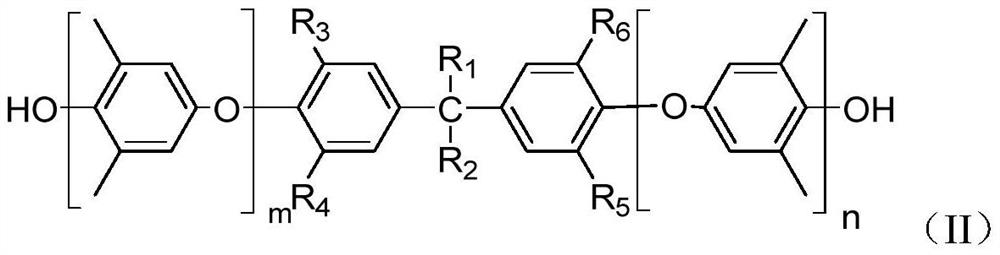
Phthalonitrile-terminated polyphenyl ether and preparation method and application thereof
A technology of phthalonitrile and double-terminated hydroxyl polyphenylene ether, which is applied in the field of polyphenylene ether modified materials and its preparation, can solve the problems of low curing crosslinking density, low activity of functional groups, limited dosing amount, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis route of the phthalonitrile-terminated polyphenylene ether prepared in this example is as follows:
[0043]
[0044] The preparation process of above-mentioned phthalonitrile-terminated polyphenylene ether comprises the following steps:
[0045] (1) Obtain a double-terminated hydroxyl polyphenylene ether solution under the action of a catalyst through oxidative copolymerization of phenolic monomers;
[0046] (11) Add 37.25g of 2,6-xylenol, 34.80g of bisphenol A and an organic solvent composed of 143.30g of toluene and 14.90g of methanol in sequence in the reaction kettle, and stir until the phenolic monomers are completely dissolved; then add 0.6g branched polyethyleneimine (Mn=600) and 0.80g cuprous bromide hydrobromic acid solution A (prepared with 0.10g cuprous oxide and 2.50g 48% hydrobromic acid in advance), after mixing uniformly 150sccm of oxygen was introduced, and the reaction was carried out at 20°C for 3 hours;
[0047] (12) After the react...
Embodiment 2
[0053] The synthesis route of the phthalonitrile-terminated polyphenylene ether prepared in this example is as follows:
[0054]
[0055] The preparation process of above-mentioned phthalonitrile-terminated polyphenylene ether comprises the following steps:
[0056] (1) Obtain a double-terminated hydroxyl polyphenylene ether solution under the action of a catalyst through oxidative copolymerization of phenolic monomers;
[0057] (11) Add 382.75g of 2,6-dimethylphenol, 29.70g of tetramethylbisphenol A and an organic solvent composed of 633.2g of toluene and 65.2g of methanol in sequence in the reaction kettle, and stir until the phenolic monomers are completely Dissolve, add 4.80g N,N-dimethylbutylamine, 1.60g branched polyethyleneimine (Mn=600) and 2.80g cuprous bromide hydrobromic acid solution (pre-prepared with 0.30g cuprous oxide and 2.50 g 48% hydrobromic acid), mix well and feed oxygen at 150 sccm, react at 60°C for 1 hour;
[0058] (12) After the reaction, add 40ml...
Embodiment 3
[0063] The synthesis route of the phthalonitrile-terminated polyphenylene ether prepared in this example is as follows:
[0064]
[0065] The preparation process of above-mentioned phthalonitrile-terminated polyphenylene ether comprises the following steps:
[0066] (1) Obtain a double-terminated hydroxyl polyphenylene ether solution under the action of a catalyst through oxidative copolymerization of phenolic monomers;
[0067] (11) Add 133.50g of 2,6-dimethylphenol, 24.50g of bisphenol F and an organic solvent composed of 430g of toluene and 43.2g of methanol to the reaction kettle in sequence, and stir until the phenolic monomers are completely dissolved; then add 4.50g N,N-dimethylbutylamine, 1.50g branched polyethyleneimine (Mn=600) and 2.80g cuprous bromide hydrobromic acid solution (pre-prepared with 0.30g cuprous oxide and 2.50g 48% Hydrobromic acid preparation), after mixing evenly, feed oxygen at 150 sccm, and react at 40°C for 2 hours;
[0068] (12) After the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com