Soft chemical synthesis method for preparing sodium ion solid electrolyte
A technology of solid electrolyte and synthesis method, which is applied in the field of soft chemical synthesis of sodium ion solid electrolyte, which can solve the problems of high cost, collapse of crystal structure, and inability to obtain pure phase exchange products, so as to reduce difficulty and avoid damage Effects of sexual influence, variety and quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
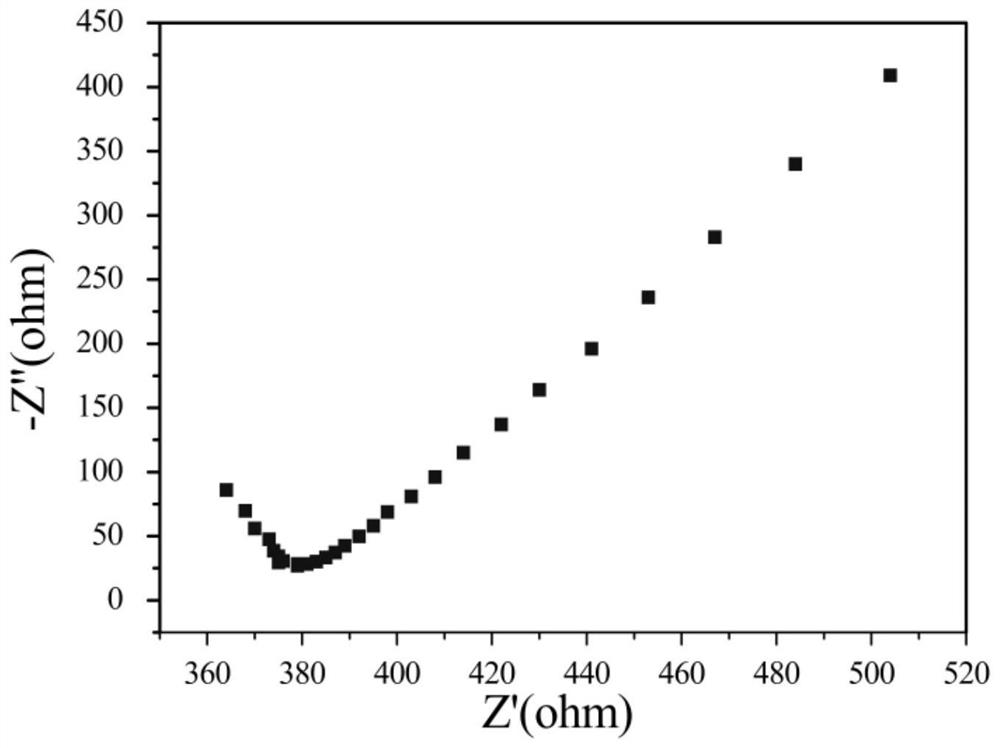
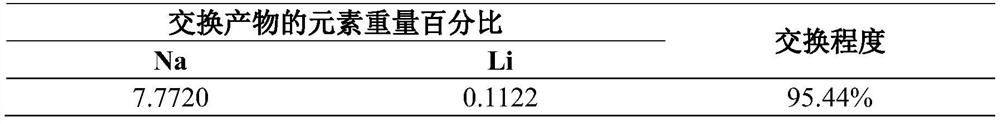
Image
Examples
Embodiment 1
[0027] Weigh n-butyl titanate (C 4 h 9 O) 4 Ti, Al(NO 3 ) 3 9H 2 O, 85 wt% H 3 PO 4 (inwater) and 5mol% excess CH 3 COOLi. Will (C 4 h 9 O) 4 Ti was fully hydrolyzed by adding deionized water, and centrifuged with ethanol to remove the organic matter produced by hydrolysis. Then, add the white precipitate obtained by hydrolysis into 2M aqueous oxalic acid solution. After the precipitate is completely dissolved, add the rest of the raw materials and heat and stir at 80°C to obtain a clear and transparent solution. Drying in an oven overnight gave a white precipitate. The precipitate was heat-treated at 400°C for 2h, then ball milled at 450r / min for 12h with a ball mill, and finally calcined at 750°C for 12h to obtain Li 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 White powder.
[0028] Weigh Li with a molar ratio of 1:40 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 The powder and anhydrous sodium acetate were placed in a mortar and mixed evenly, then the mixed powder was poured into an a...
Embodiment 2
[0034] In a dry room, weigh Li with a molar ratio of 1:40 7 La 3 Zr 2 o 12 The powder and anhydrous sodium acetate were placed in a mortar and mixed evenly, then the mixed powder was poured into an alumina crucible, and heat treated at 240°C for 2h. Afterwards, centrifuge four times with diethyl ether to remove sodium acetate, then centrifuge four times with absolute ethanol to remove lithium acetate produced by ion exchange reaction, and finally dry the product in a drying room at 60-80°C. The above steps were repeated several times until the ion exchange reaction reached equilibrium.
Embodiment 3
[0036] Weigh Li with a molar ratio of 1:40 0.5 La 0.5 TiO 3 The powder and anhydrous sodium acetate were placed in a mortar and mixed evenly, then the mixed powder was poured into an alumina crucible, and heat treated at 240°C for 2h. Afterwards, centrifuge five times with deionized water to remove excess sodium acetate and lithium acetate produced by exchange, and finally remove water by centrifuging with ethanol, and dry the product at 60-80°C. The above steps were repeated several times until the ion exchange reaction reached equilibrium.
[0037] The principle of the present invention is that the crystal structure of the lithium-ion solid electrolyte is composed of an anion skeleton and lithium ions, and the lithium ions in the skeleton have high mobility. Under the driving force of temperature and concentration difference, they can interact with sodium ions The sodium ions of the exchanger rapidly generate Li + / Na + In the exchange reaction, lithium ions are extract...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com