Catalyst for preparing isopropylbenzene as well as preparation and application of catalyst
A technology of catalyst and cumene, which is applied in the field of catalysts for preparing cumene, and can solve problems such as poor stability, low catalyst activity and selectivity, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] 1. Catalyst preparation
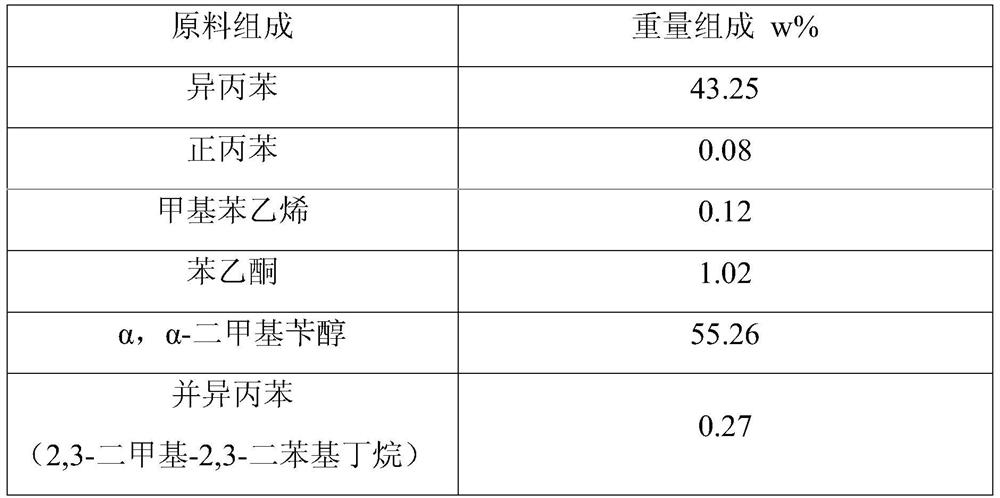
[0083] Mix 1 liter of alumina with 600 grams of phosphoric acid aqueous solution containing 8.0 grams of P, dry at 110°C for 8 hours, and calcinate at 400°C for 4 hours to obtain a catalyst carrier.
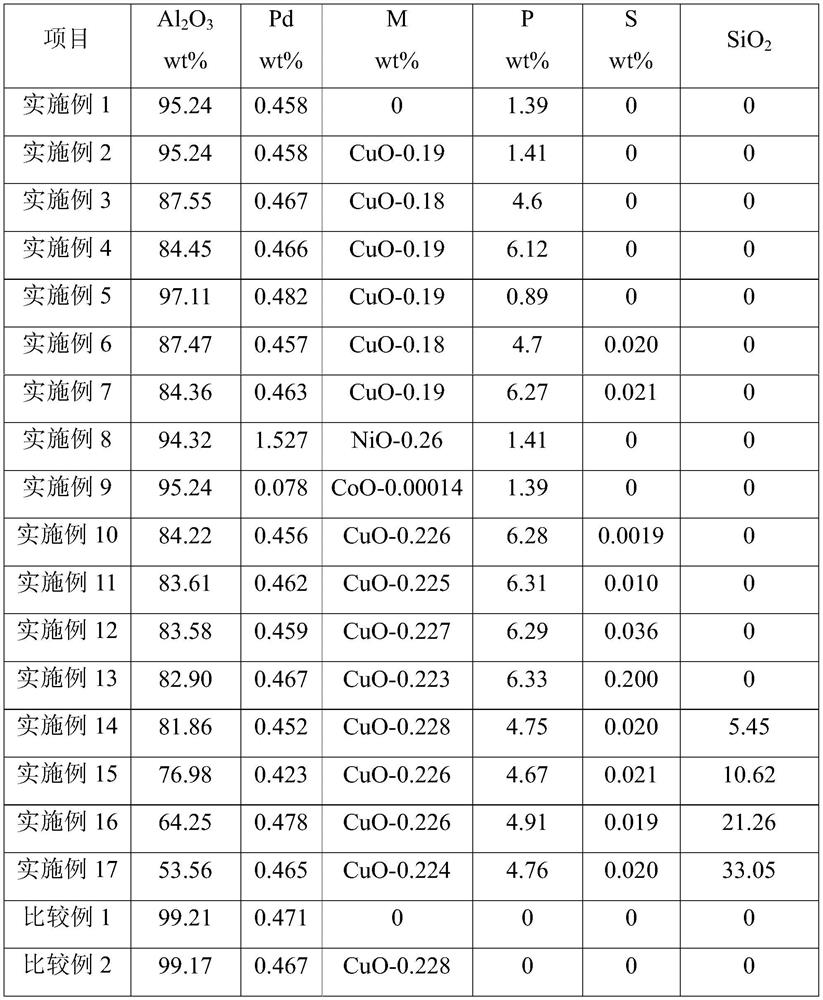
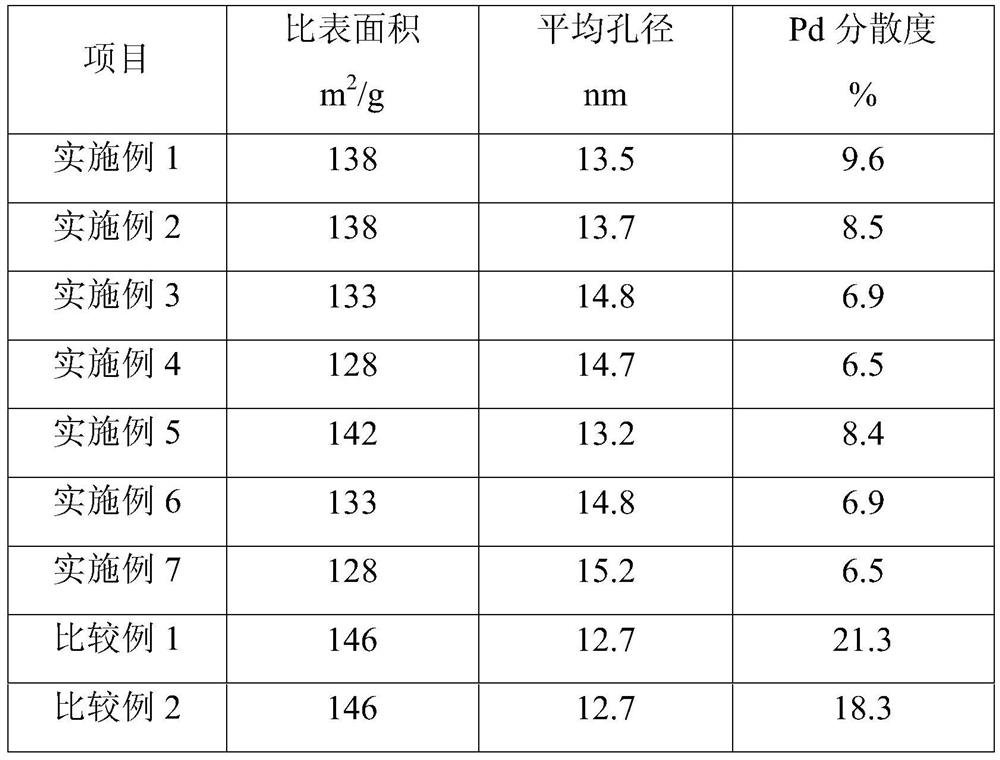
[0084] Mix 1 liter of the above-mentioned support with 2000 g of aqueous chloropalladium acid solution containing 3.0 g of palladium, dry at 110° C. for 8 hours, and roast at 500° C. for 4 hours to obtain the oxidized palladium-based catalyst precursor I. The above-mentioned oxidized palladium-based catalyst precursor I was reduced with hydrogen for 4 hours, the reduction temperature was 250°C, and the hydrogen volume space velocity was 100 hours -1 , to obtain a palladium-based catalyst, the main composition and properties of the catalyst are shown in Table 2 and Table 3.
[0085] 2. Catalyst evaluation
[0086] The hydrogenation operation is carried out in a fixed-bed reactor, and the catalyst prepared above is filled in the reactor, and the h...
Embodiment 2
[0095] 1. Catalyst preparation
[0096] Mix 1 liter of alumina with 600 grams of phosphoric acid aqueous solution containing 8.0 grams of P, dry at 110°C for 8 hours, and calcinate at 400°C for 4 hours to obtain a catalyst carrier.
[0097] Mix 1 liter of the above carrier with 2,000 grams of chloropalladic acid-copper nitrate aqueous solution containing 3.0 grams of palladium and 1.0 grams of copper, dry at 110°C for 8 hours, and roast at 500°C for 4 hours to obtain the oxidized state palladium-based catalyst precursor I .
[0098] The above-mentioned oxidized palladium-based catalyst precursor I was reduced with hydrogen for 4 hours, the reduction temperature was 250°C, and the hydrogen volume space velocity was 100 hours -1 , to obtain a palladium-based catalyst.
[0099] The main composition and properties of the catalyst are shown in Table 2 and Table 3.
[0100] 2. Catalyst evaluation
[0101] The hydrogenation operation is carried out in a fixed-bed reactor, and the...
Embodiment 3
[0110] 1. Catalyst preparation
[0111] Mix 1 liter of alumina with 600 grams of phosphoric acid aqueous solution containing P27 grams, dry at 110°C for 8 hours, and roast at 400°C for 4 hours to obtain a catalyst carrier.
[0112] Mix 1 liter of the above carrier with 2,000 grams of chloropalladic acid-copper nitrate aqueous solution containing 3.0 grams of palladium and 1.0 grams of copper, dry at 110°C for 8 hours, and roast at 500°C for 4 hours to obtain the oxidized state palladium-based catalyst precursor I .
[0113] The above-mentioned oxidized palladium-based catalyst precursor I was reduced with hydrogen for 4 hours, the reduction temperature was 250°C, and the hydrogen volume space velocity was 100 hours -1 , to obtain a palladium-based catalyst.
[0114] The main composition and properties of the catalyst are shown in Table 2 and Table 3.
[0115] 2. Catalyst evaluation
[0116] The hydrogenation operation is carried out in a fixed-bed reactor, and the catalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com