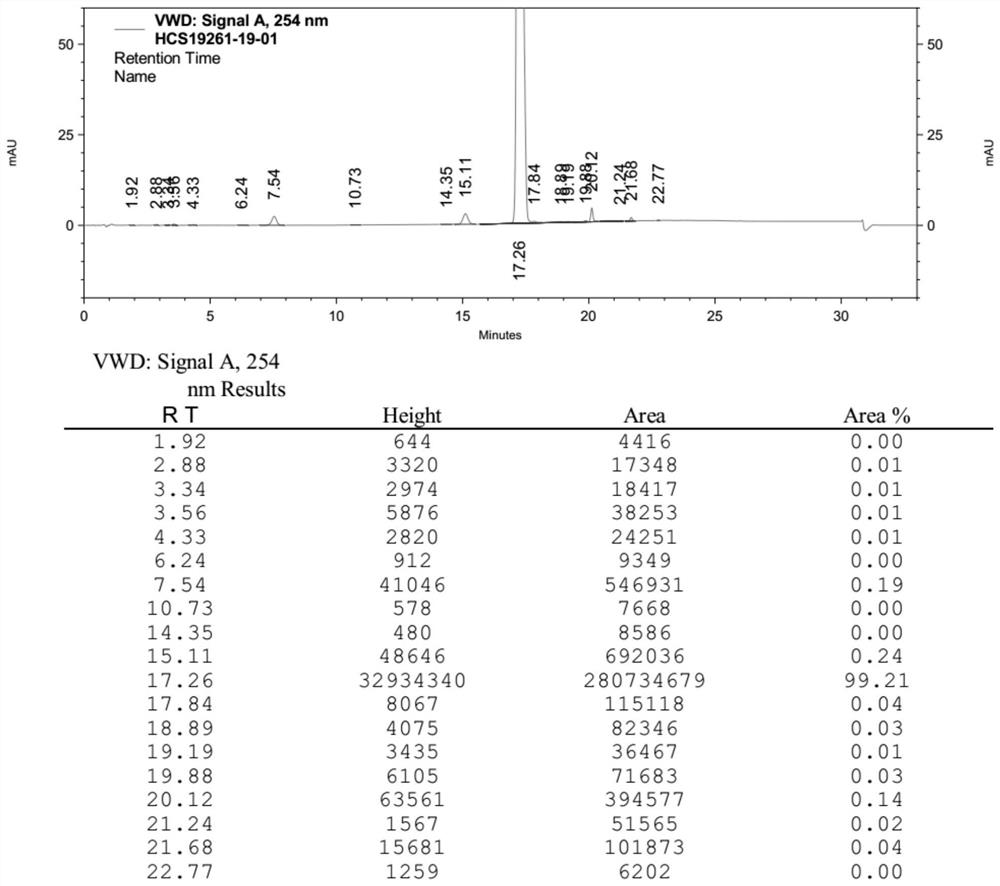
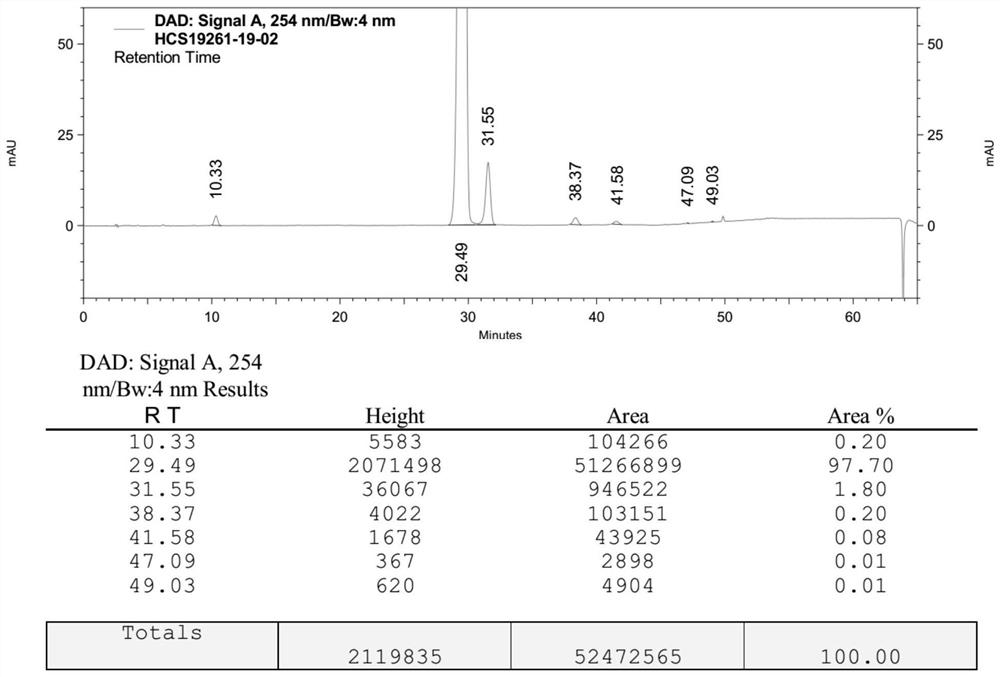
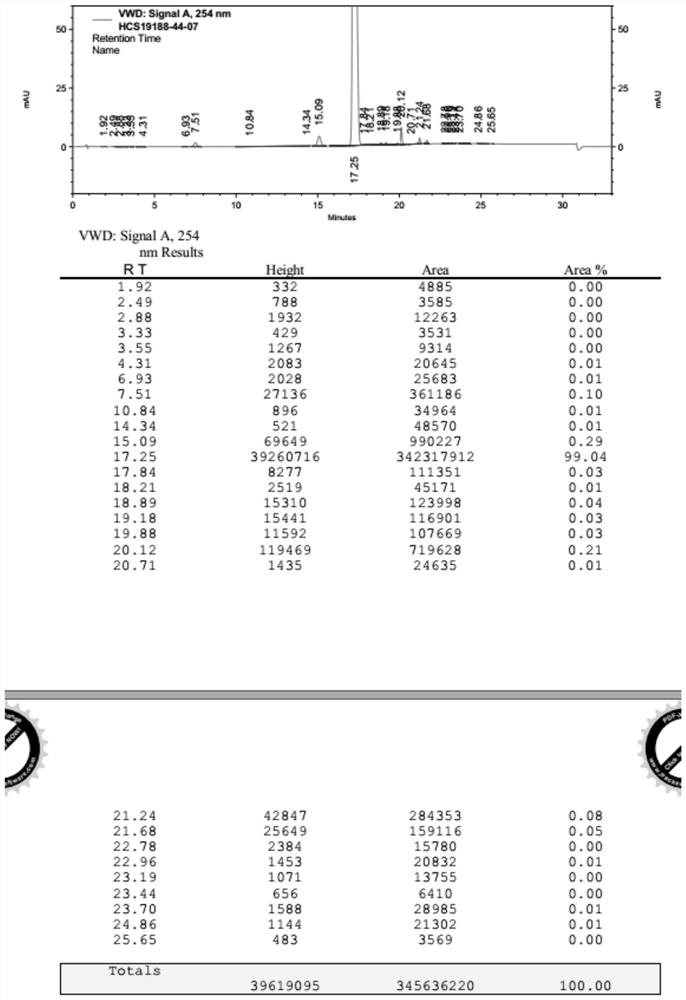
Preparation method of sacubitril valsartan sodium
A sodium hydroxide and compound technology, applied in the field of medicine and chemical industry, can solve the problems of inconvenient solvent recovery, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of embodiment 1 compound SAC04
[0083]
[0084] At room temperature, 100mL of ethanol, ruthenium reagent and iron reagent were stirred in a one-mouth bottle for 30min, and stirred evenly. S309A03 (50 g), ruthenium reagent (40 mg) and iron reagent (65 mg) solutions and 400 mL of ethanol were sequentially added into the reaction kettle. After replacing three times with nitrogen gas, replace it with hydrogen gas three times, then charge hydrogen pressure to 3.0MPa, and raise the temperature to 65-80°C for reaction. After 24 hours of reaction, the heating was turned off to lower the temperature, and the reaction solution of BPA08 obtained was directly used in subsequent reactions.
[0085] The room temperature was 27°C, and the above BPA08 solution was added to a 1L four-neck flask at room temperature, and 18.64g SOCl2 was added dropwise. After the drop was complete, it was stirred at 45°C, and the transition state product was detected by HPLC <0.5%, an...
Embodiment 2
[0087] The preparation of embodiment 2 compound SAC04
[0088] At room temperature, 100 mL of ethanol, ruthenium reagent (20 mg) and iron reagent (32.5 mg) were stirred in a one-mouth bottle for 30 min, and stirred evenly. Add S309A03 (50g), ruthenium reagent and iron reagent solution and 400mL ethanol to the reaction kettle successively. After replacing three times with nitrogen gas, replace it with hydrogen gas three times, then charge hydrogen pressure to 3.0MPa, and raise the temperature to 65-80°C for reaction. After 24 hours of reaction, the heating was turned off to cool down, and the reaction solution of BPA08 was obtained, which was directly used in subsequent reactions.
[0089] At room temperature 27°C, add the above BPA08 solution to a 1L four-neck flask at room temperature, add 23.3g SOCl2 dropwise, after the drop is complete, place it at 65°C and stir at 65°C to detect transition state products < 0.5%, then stop the reaction, and the reaction solution is at 50-6...
Embodiment 3
[0091] The preparation of embodiment 3 compound SAC04
[0092] At room temperature, 100 mL of ethanol, ruthenium reagent (20 mg) and iron reagent (32.5 mg) were stirred in a one-mouth bottle for 30 min, and stirred evenly. Add S309A03 (50g), ruthenium reagent and iron reagent solution and 400mL ethanol to the reaction kettle sequentially. After replacing three times with nitrogen gas, replace it with hydrogen gas three times, then charge hydrogen pressure to 3.0MPa, and raise the temperature to 65-80°C for reaction. After 24 hours of reaction, the heating was turned off to cool down, and the reaction solution of BPA08 was obtained, which was directly used in subsequent reactions.
[0093] At room temperature of 27°C, add the above-mentioned BPA08 solution to a 1L four-neck flask at room temperature, add 23.3g of SOCl2 dropwise, after the drop is complete, place it at 65°C and stir for 1 hour, then take a sample and send it to HPLC to detect that the transition state product i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com