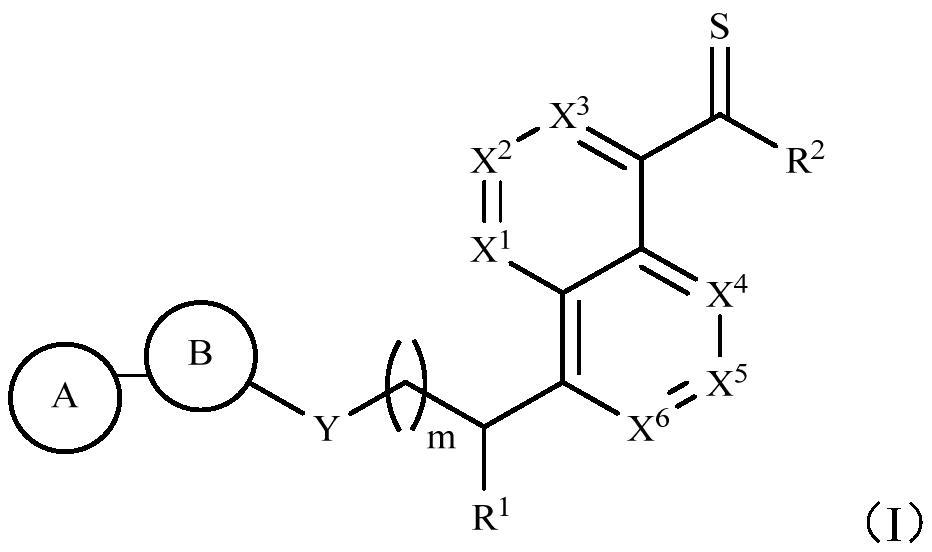
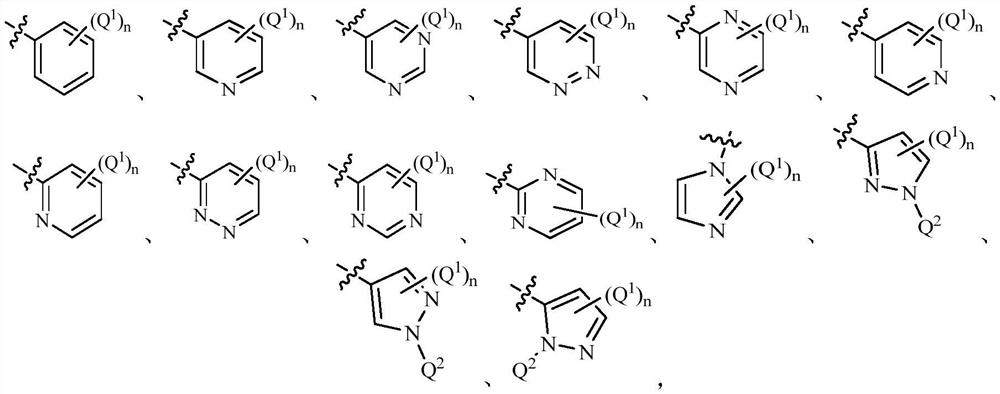
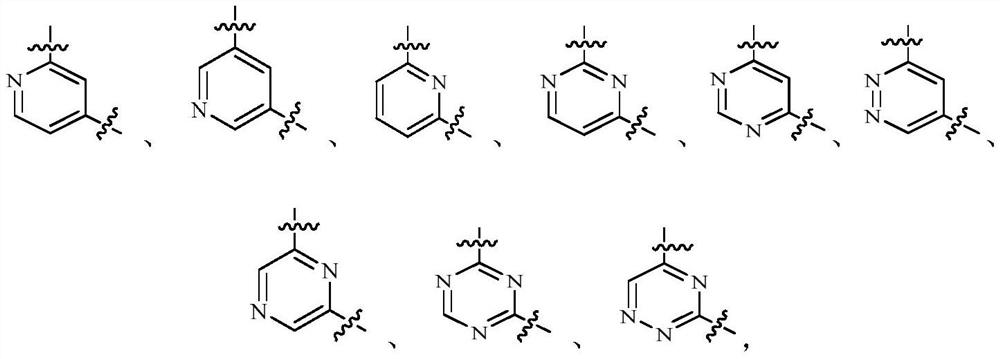
DNA-PK inhibitor
A technology based on alkylation and selection, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve problems such as cell death and apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0161] 1 Preparation examples of compounds of the present invention
[0162] In the preparation examples, the meanings represented by the abbreviations are as follows:
[0163] KOH: potassium hydroxide EA: ethyl acetate DCM: dichloromethane
[0164] DMAP: 4-Dimethylaminopyridine Na 2 SO 4 : Sodium sulfate TFA: Trifluoroacetic acid
[0165] DMF: N,N-Dimethylformamide THF: Tetrahydrofuran Pd / C: Palladium / Carbon catalyst
[0166] MeOH: Methanol EtOH: Ethanol Tf 2 O: Trifluoromethanesulfonic anhydride
[0167] EDCI: 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride DIEA: N,N-Diisopropylethylamine
[0168] Lawson's reagent: 2,4-bis(p-methoxyphenyl)-1,3-dithio-diphosphetane-2,4 sulfide
[0169] HATU: 2-(7-Azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate
preparation example 1
[0170] Preparation 1: N-methyl-8-(1-((2'-methyl-[4,5'-bipyrimidin]-6-yl)amino)propan-2-yl)quinoline-4-sulfur Preparation of formamide (compound 1)
[0171] 1, Preparation of 8-(3-((tert-butoxycarbonyl)amino)prop-1-en-2-yl)quinoline-4-carboxylic acid
[0172]
[0173] Dissolve tert-butyl (2-(4-cyanoquinolin-8-yl)allyl)carbamate (1.2g, 3.9mmol), KOH (0.87g, 15.5mmol) in ethanol (30mL), water (10 mL), react at 110° C. for 10 h. After the reaction was completed, adjust the pH to 5-6 with dilute hydrochloric acid, add water (20mL), extract three times with EA (3×30mL), combine the organic phases, wash over anhydrous Na 2 SO 4 Dried, filtered, and spin-dried to obtain 770 mg of the product, with a yield of 60.3%.
[0174] 2. Preparation of (2-(4-(methylcarbamoyl)quinolin-8-yl)allyl)carbamate tert-butyl ester
[0175]
[0176] 8-(3-((tert-butoxycarbonyl)amino)prop-1-en-2-yl)quinoline-4-carboxylic acid (770mg), methylamine hydrochloride (317mg, 4.70mmol), EDCI (893mg, 4.70...
preparation example 2
[0191] Preparation 2: (R)-N-methyl-8-(1-(((2'-methyl-[4,5'-pyrimidin]]-6-yl)amino)propan-2-yl)quinone Preparation of phenoline-4-thiocarboxamide (compound 1-1)
[0192] 1. Preparation of tert-butyl (2-(4,4,5,5-tetramethyl-1,3,2-dioxabororan-2-yl) allyl) carbamate
[0193]
[0194] At 0°C, add N-tert-butoxycarbonylaminopropyne (150.0g, 967.7mmol), B 2 (Pin) 2 (300.0 g, 1181.1 mmol), CuCl (10.0 g, 100.8 mmol), t-BuONa (15.0 g, 156.1 mmol), and P(t-Bu) 3 (25.0 g, 123.6 mmol) was slowly added dropwise to MeOH (75.0 mL, 1875.0 mmol) in 3.0 L of toluene suspension. After the dropwise addition, the system was warmed up to 20° C. and stirred for 16 h. After silica gel column chromatography (petroleum ether: ethyl acetate = 5:1), the crude compound (330.0 g) was obtained, which was directly used in the next step.
[0195] 2. Preparation of (2-(4-hydroxyquinolin-8-yl) allyl) tert-butyl carbamate
[0196]
[0197] Will contain 4-hydroxy-8-bromoquinoline (150.0g, 669.6mmol), te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com