Conjugated microporous polymer based on 2,4,6-tri(4-formylphenyl)-1,3,5-triazine and preparation method of conjugated microporous polymer
An aldehyde-based phenyl, conjugated microporous technology, applied in the field of conjugated microporous polymers, can solve the problems of inability to meet the needs of diversified CMPs, few types of CMPs, etc., achieve large-scale production, and the preparation method is simple and easy. row effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The invention provides a method for preparing a conjugated microporous polymer based on 2,4,6-tris(4-formylphenyl)-1,3,5-triazine, comprising the following steps:
[0040] Mix the first monomer, the second monomer and an organic solvent, and react in a protective atmosphere to obtain a co- conjugated microporous polymers;
[0041] The first monomer is 2,4,6-tris(4-formylphenyl)-1,3,5-triazine, and the second monomer includes thiooxamide or symmetric indacene-1 ,3,5,7(2H,6H)-tetraketone.
[0042] In the present invention, the molar ratio of the first monomer to the second monomer is preferably 2:(2.8-3.2), more preferably 2:3.
[0043] In the present invention, the type of organic solvent and specific reaction conditions are preferably selected according to the specific type of the second monomer.
[0044] In the present invention, when the second monomer is thiooxamide, the organic solvent preferably includes N,N-dimethylformamide, N,N-dimethylacetamide and dioxane ...
Embodiment 1
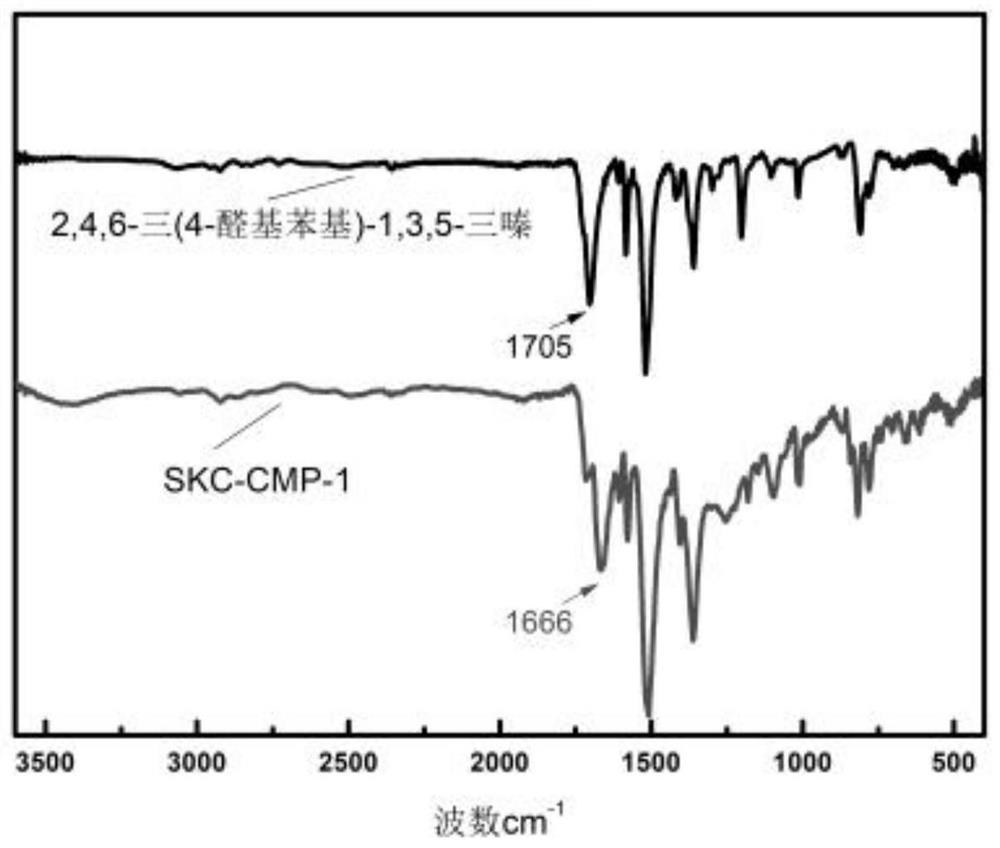
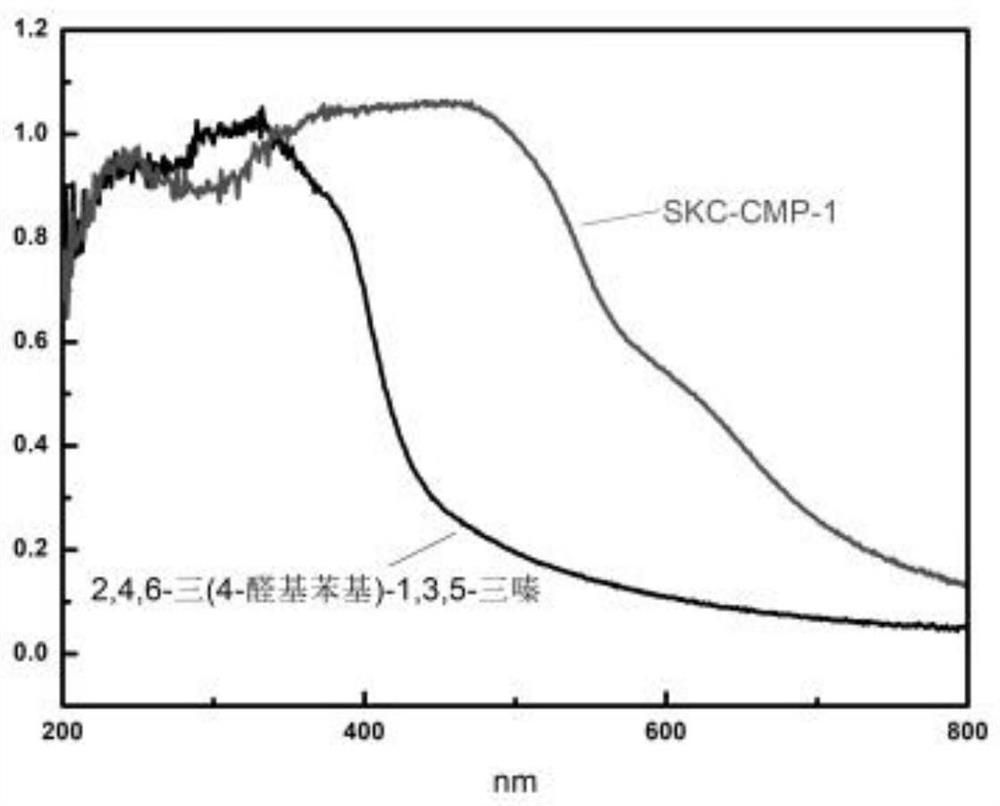
[0067] Thiooxamide (0.3606g, 3mmol), 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (0.7868g, 2mmol) and N,N-dimethyl Formamide (10 mL) was added to the beaker, the resulting mixture was ultrasonically dispersed and then poured into the hydrothermal synthesis reaction kettle, and nitrogen gas was passed for 5 minutes, and the hydrothermal synthesis reaction kettle was sealed and placed in a constant temperature drying oven at 160 °C The polymerization reaction was carried out under the conditions for 24 hours; after the reaction was completed, it was cooled to room temperature, the obtained product system was suction filtered, the obtained solid material was washed with absolute ethanol, and dried, and the obtained brown-yellow product was based on 2,4,6-tris(4- The conjugated microporous polymer of aldehyde phenyl)-1,3,5-triazine (abbreviated as SKC-CMP-1, the specific structural formula is shown in formula I), the yield is 0.7932g, and the yield is 72%.
[0068]
Embodiment 2
[0070] The preparation of symmetric indacene-1,3,5,7(2H,6H)-tetraketone comprises the following steps:
[0071] Mix pyromellitic dianhydride (20.0005g, 0.091mol), ethyl acetoacetate (35mL, 0.275mol) and triethylamine (112mL, 1.1mol), heat the oil bath to 60°C, and add ethyl Acid anhydride (300mL), heat up to 100°C, keep warm for 2h; cool to room temperature after the reaction, then cool at 0°C for 12h, a brown precipitate appears, vacuum filter the resulting material, and wash with acetic anhydride (20mL) and anhydrous diethyl ether (20mL) were washed 3 times, and the first orange solid was obtained after drying;
[0072] The first orange solid (6.0005g, 10.5mmol) was dissolved in distilled water (500mL) to form a dark orange solution, and concentrated sulfuric acid (6mL, 98wt%) was added to the dark orange solution under ice-water bath conditions, A solid precipitated, filtered under reduced pressure and washed with absolute ethanol, and dried to obtain a second orange solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com