Preparation method of levofloxacin eye drops
A technology of levofloxacin and eye drops, applied in the field of preparation of levofloxacin eye drops, can solve problems such as inapplicability to sensitive people, damage to epithelial cells, and potential safety hazards, so as to relieve symptoms of eye discomfort, promote healing, and enhance use effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
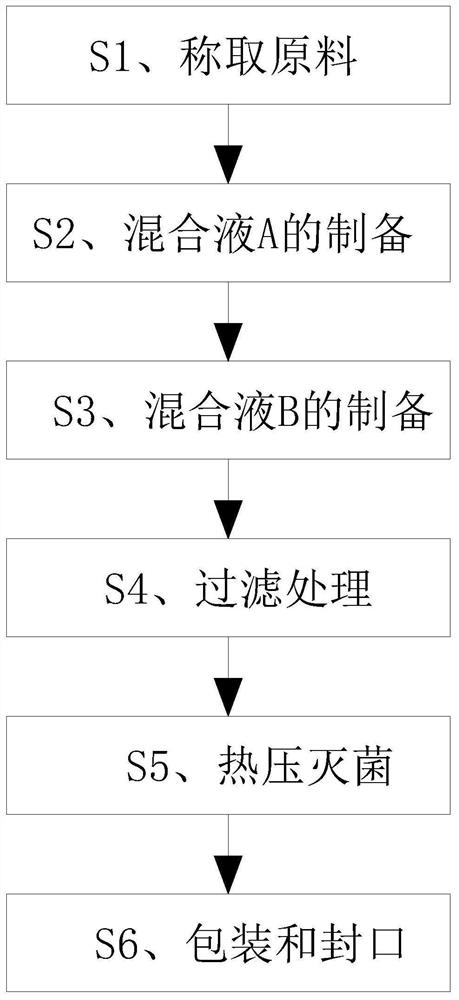
[0027] Please refer to figure 1 Shown, the invention discloses a kind of preparation method of levofloxacin eye drops, comprises the following steps:
[0028] S1. Weighing raw materials: 0.46 parts of levofloxacin, 4.46 parts of osmotic pressure regulator, 3 parts of pH regulator, 80 parts of water for injection, 2 parts of compound thickener, 8 parts of metal ion complexing agent and 0.05 part of bacteriostatic agent;
[0029] S2. Preparation of mixed solution A: Weigh levofloxacin into an appropriate amount of water for injection, stir and dissolve at 35-40°C to obtain mixed solution A, and set aside;
[0030] S3. Preparation of mixed solution B: add osmotic pressure regulator, metal ion complexing agent, compound thickener and pH regulator to the mixed solution A prepared in step S2, stir and mix, and dilute to 100mL with water for injection , the metal ion complexing agent is disodium edetate, the weight ratio of the metal ion complexing agent to levofloxacin is 1:0.01, t...
Embodiment 2
[0035] Please refer to figure 1 Shown, the invention discloses a kind of preparation method of levofloxacin eye drops, comprises the following steps:
[0036] S1. Weigh raw materials: 0.48 parts of levofloxacin, 4.46 parts of osmotic pressure regulator, 3-5 parts of pH regulator, 85 parts of water for injection, 2.5 parts of compound thickener, 9 parts of metal ion complexing agent and 0.05 parts of antibacterial agent share;
[0037] S2. Preparation of mixed solution A: Weigh levofloxacin into an appropriate amount of water for injection, stir and dissolve at 35-40°C to obtain mixed solution A, and set aside;
[0038] S3. Preparation of mixed solution B: add osmotic pressure regulator, metal ion complexing agent, compound thickener and pH regulator to the mixed solution A prepared in step S2, stir and mix, and dilute to 100mL with water for injection , the metal ion complexing agent is sodium gluconate, the weight ratio of the metal ion complexing agent to levofloxacin is 1...
Embodiment 3
[0043] Please refer to figure 1 Shown, the invention discloses a kind of preparation method of levofloxacin eye drops, comprises the following steps:
[0044] S1. Weighing raw materials: 0.51 parts of levofloxacin, 8.23 parts of osmotic pressure regulator, 5 parts of pH regulator, 30 parts of water for injection, 3 parts of compound thickener, 10 parts of metal ion complexing agent and 0.1 part of antibacterial agent;
[0045] S2. Preparation of mixed solution A: Weigh levofloxacin, add it to an appropriate amount of water for injection, stir and dissolve at 40°C to obtain mixed solution A, and set aside;
[0046]S3. Preparation of mixed solution B: add osmotic pressure regulator, metal ion complexing agent, compound thickener and pH regulator to the mixed solution A prepared in step S2, stir and mix, and dilute to 100mL with water for injection , the metal ion complexing agent is disodium edetate, the weight ratio of the metal ion complexing agent to levofloxacin is 1:0.50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com