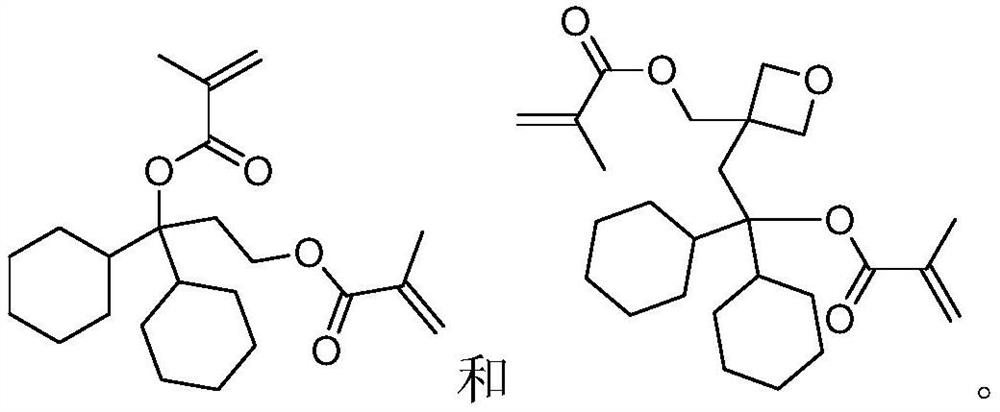
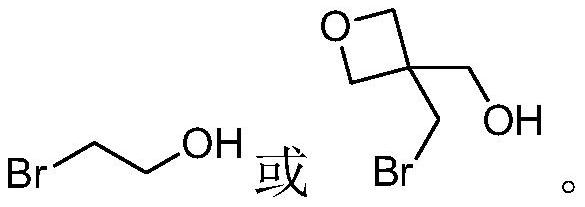
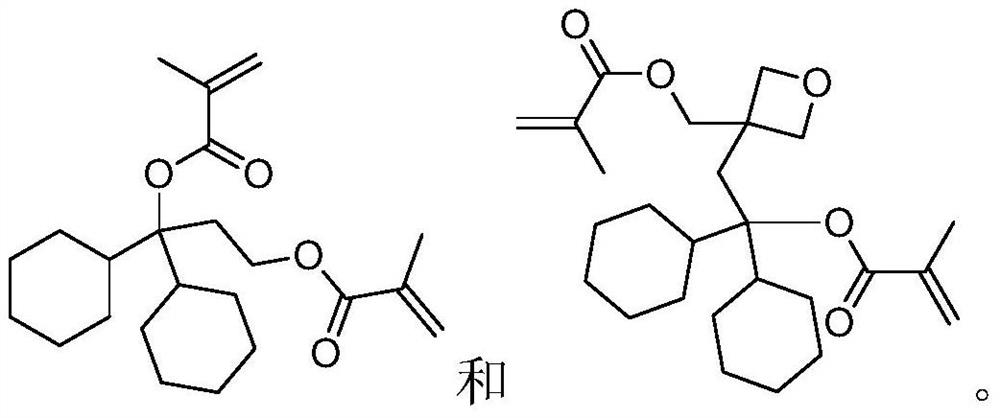
Degradable resin monomer synthesized from dicyclohexylketone and preparation method of degradable resin monomer
A technology of dicyclohexyl ketone and resin monomers, which is applied in the field of degradable resin monomers and its preparation, can solve the problem of low resolution of photolithography patterns, and achieve the effect of simple synthesis route and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Preparation of compound 1-2:
[0029] Dissolve tert-butyldimethylsilyl chloride (13g, 86.3mmol) in tetrahydrofuran (50g), and then add the above mixture dropwise to 2-bromoethanol 1-1 (10g, 80.0 mmol), triethylamine (10g, 98.8mmol) and 4-dimethylaminopyridine (1g, 8.2mmol) in tetrahydrofuran (100g) solution, stirred for 3 hours. The resulting suspension was filtered to obtain a filtrate, which was concentrated and purified by vacuum distillation to obtain compound 1-2 (15.5 g, 64.8 mmol, molar yield 81.0%).
[0030] Preparation of Compound 1-3:
[0031] Compound 1-2 (13g, 54.3mmol) was dissolved in ether (100g), and under nitrogen protection, the above mixture was added dropwise to ether (30g) containing magnesium (1.3g, 54.2mmol), at 25°C Next, stir for 1 hour. Then dicyclohexyl ketone (10 g, 51.5 mmol) was dissolved in ether (80 g), dropped into the above mixture, and stirred for 3 hours. The reaction solution was quenched, then filtered, and the filtr...
Embodiment 2
[0037]
[0038] Preparation of compound 2-2:
[0039] Dissolve tert-butyldimethylsilyl chloride (10g, 66.3mmol) in tetrahydrofuran (50g), and then add the above mixture dropwise to 3-bromomethyl-3-hydroxymethyl -1-oxetane 1-1 (10g, 55.2mmol), triethylamine (8g, 79.1mmol) and 4-dimethylaminopyridine (1g, 8.2mmol) in tetrahydrofuran (100g) solution, stirring 3 hours. The resulting suspension was filtered to obtain a filtrate, which was concentrated and purified by vacuum distillation to obtain compound 2-2 (13 g, 44.0 mmol, molar yield 79.7%).
[0040] Preparation of compound 2-3:
[0041] Compound 2-2 (13g, 44.0mmol) was dissolved in ether (100g), and under nitrogen protection, the above mixture was added dropwise to ether (30g) containing magnesium (1.2g, 50mmol), at 25°C , and stirred for 1 hour. Then dicyclohexyl ketone (8 g, 41.2 mmol) was dissolved in ether (80 g), dropped into the above mixture, and stirred for 3 hours. The reaction solution was quenched, then fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com