Disulfide bond bridged docetaxel-fatty acid prodrugs and self-assembled nanoparticles thereof
A technology of self-assembled nanoparticles and disulfide bond bridging, which is applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., to achieve the effects of easy surface modification, improved tolerance and compliance, and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
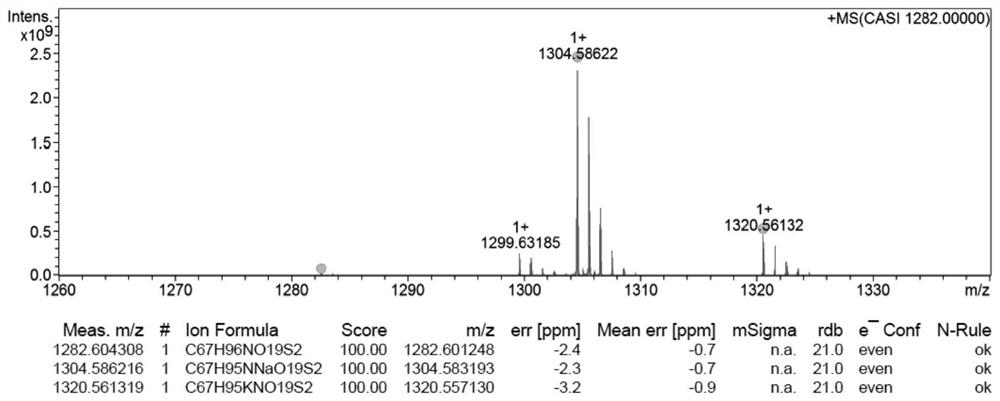
[0031] Embodiment 1: the synthesis of the docetaxel-stearic acid prodrug (DTX-S-S-SA) of disulfide bond bridge
[0032] Measure excessive ethylene glycol and place it in a 50mL three-necked flask, add an appropriate amount of p-toluenesulfonic acid into the three-necked flask, and return the spherical condenser to N 2 Protected and heated to 110°C, the stearic acid solution dissolved in toluene was slowly dripped into the reaction bottle through a constant pressure dropping funnel, and reacted for 2 hours. After the reaction, the layers were left to stand, extracted three times with toluene, the toluene layers were combined, and then saturated NaHCO 3 The solution was washed until neutral, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, separated and purified to obtain 2-hydroxyethyl stearate. 2,2'-dithiodiacetic anhydride with CH 2 Cl 2 Dissolve and transfer to a 100mL eggplant-shaped bottle, add HOBt, EDCI and 2-hydroxyethyl stearate, N 2 Cool down ...
Embodiment 2
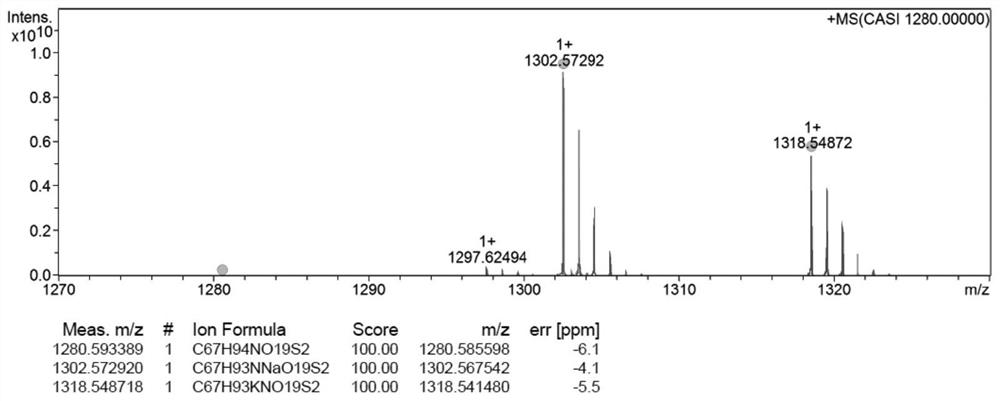
[0034] Embodiment 2: the synthesis of the docetaxel-oleic acid prodrug (DTX-S-S-OA) of disulfide bond bridge
[0035] Measure excessive ethylene glycol and place it in a 50mL three-necked flask, add an appropriate amount of p-toluenesulfonic acid into the three-necked flask, and return the spherical condenser to N 2 Protected, heated to 110°C, and the oleic acid solution dissolved in toluene was slowly dripped into the reaction bottle through a constant pressure dropping funnel, and reacted for 2 hours, and the reaction was complete as monitored by TLC. After the reaction is over, let the layers stand, extract with toluene until the ethylene glycol layer TLC has no product point, combine the toluene layers, and then use saturated NaHCO 3 The solution was washed until neutral, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, separated and purified to obtain oleic acid-2-hydroxyethyl ester. 2,2'-dithiodiacetic anhydride with CH 2 Cl 2 Dissolve and transfe...
Embodiment 3
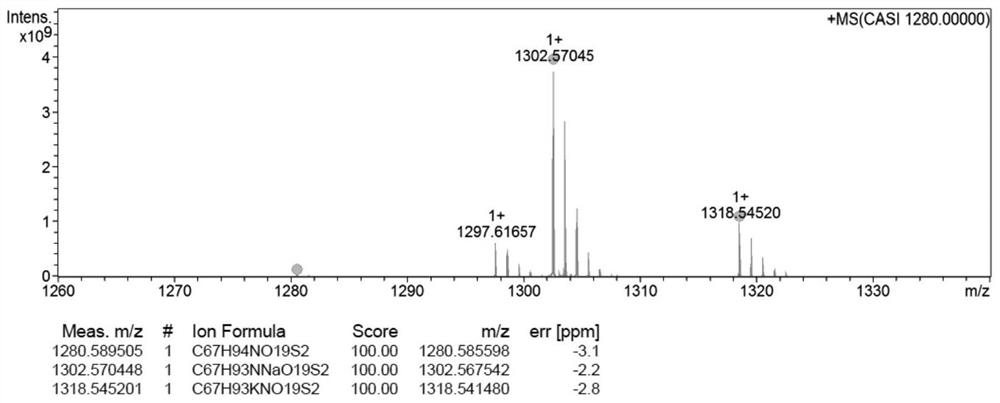
[0037] Example 3: Synthesis of disulfide bridged docetaxel-elaidic acid prodrug (DTX-S-S-EA)
[0038] Measure excessive ethylene glycol and place it in a 50mL three-necked flask, add an appropriate amount of p-toluenesulfonic acid into the three-necked flask, and return the spherical condenser to N 2 Protected, heated to 110°C, and the solution of elaidic acid dissolved in toluene was slowly dripped into the reaction bottle through a constant pressure dropping funnel, and reacted for 2 hours, and the reaction was complete as monitored by TLC. After the reaction is over, let the layers stand, extract with toluene until the ethylene glycol layer TLC has no product point, combine the toluene layers, and then use saturated NaHCO 3 The solution was washed until neutral, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, separated and purified to obtain elaidic acid-2-hydroxyethyl ester. 2,2'-dithiodiacetic anhydride with CH 2 Cl 2 Dissolve and transfer to a 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com