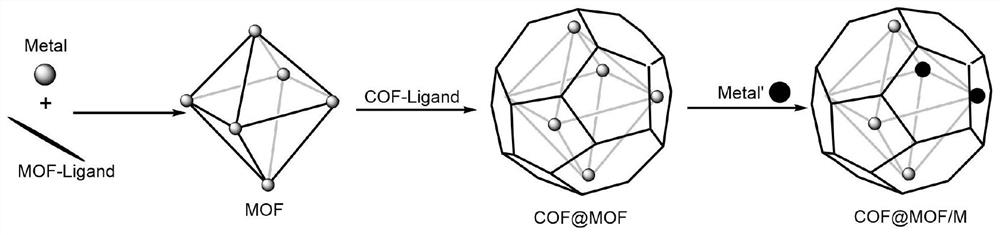
Ion exchange type COF-coated MOF/M composite material and preparation method thereof
A composite material and ion exchange technology, applied in the field of ion exchange COF@MOF/M composite material and its preparation, to achieve the effect of improving catalytic performance and degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
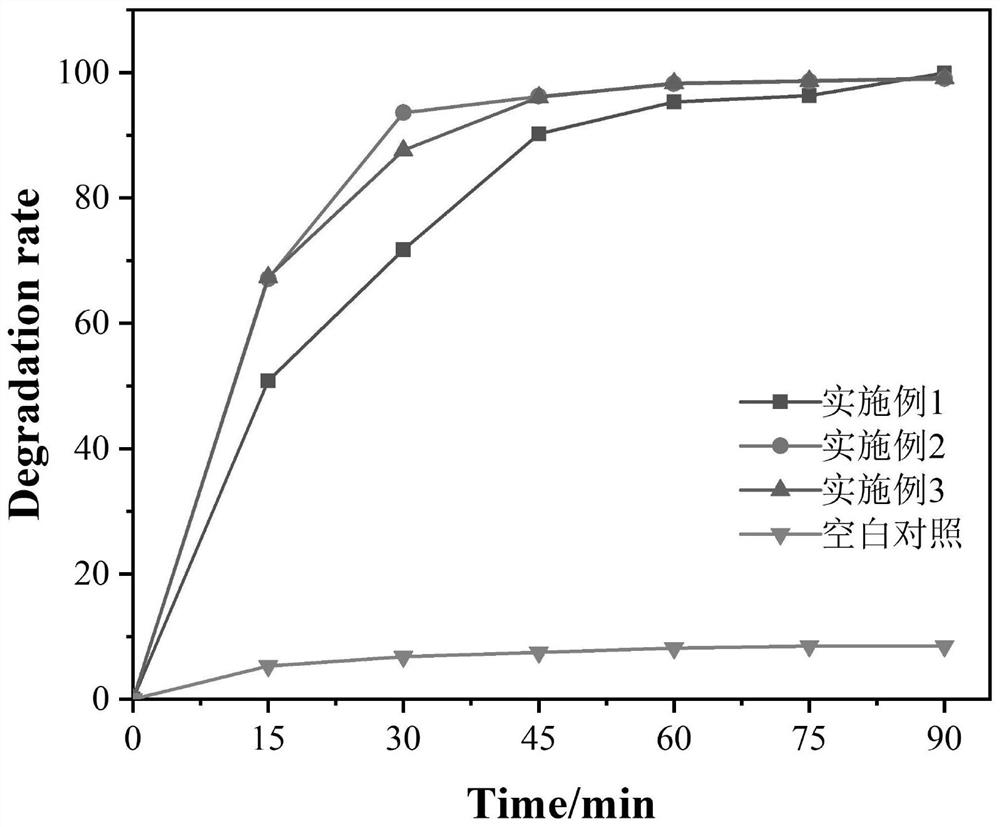
Image
Examples
Embodiment 1
[0024] Synthesis of COF1@UiO-66 / Ti(Ⅳ) Composite
[0025] 1) Synthesis of UiO-66:
[0026] At room temperature, add DMF (75mL) and ZrCl in a 250mL round bottom flask 4(0.4 g,), acetic acid (2.85 mL, 850 mmol) was added to the solution during ultrasonic dispersion at 60°C. A solution of terephthalic acid (0.282 g) in DMF (25 mL) was added to the flask and finally deionized water (0.125 mL) was added. The mixed solution was tightly sealed, ultrasonically dispersed at 60°C for 10min, and heated and stirred in a constant temperature oil bath at 120°C for 24h. Then the solution was cooled to room temperature, and the precipitate was separated by centrifugation and washed with DMF (10mL) and purified three times by centrifugation. The obtained yellow solid powder was UiO-66, which was washed three times with methanol (10mL), and finally placed in an oven at 60°C. After drying for 24h, the UiO-66 sample was obtained.
[0027] 2) Synthesis of COF1@UiO-66:
[0028] At room temperat...
Embodiment 2
[0032] Synthesis of COF2@IRMOF-3 / Co composites
[0033] 1) Synthesis of IRMOF-3
[0034] Dissolve 3.0mmol of zinc nitrate hexahydrate and 0.85mmol of 2-aminoterephthalic acid in 20ml of DMF, ultrasonically disperse for 2min, then place the mixed solution in a reaction kettle, react at 110°C for 15h, and cool naturally to The product was separated by centrifugation at room temperature, washed three times with DMF and methanol, and dried in an oven at 50°C for 8 hours to obtain the IRMOF-3 sample.
[0035] 2) Synthesis of COF2@IRMOF-3
[0036] 40 mg of IRMOF-3 was dissolved in 1,2,4,5-tetrakis(4-formylphenyl)benzene (0.022 mmol) in 1,4-dioxane (2.5 mL) and stirred for 30 min until After becoming uniform, 1,3,5-tris(4-aminophenyl)benzene (TAPB, 0.02 mmol) and 30 μL acetic acid were added during ultrasonic dispersion, and ultrasonic dispersion was continued for 30 min. Then heated and stirred at 100°C for 48h, centrifuged to separate the product, washed three times with THF (te...
Embodiment 3
[0040] Synthesis of COF3@ZIF-67 / Fe
[0041] 1) Synthesis of ZIF-67
[0042] At room temperature, 1 mmol of Co(NO 3 )2·6H 2 O and 4mmol of 2-methylimidazole were dissolved in 25ml of ethanol, and ultrasonically dispersed for 30min. Then the two solutions were mixed, reacted in an oil bath at 120°C for 4 hours, cooled naturally to room temperature, centrifuged to separate the product, washed with ethanol and acetone three times, and dried in an oven at 85°C for 6 hours to obtain the ZIF-67 product.
[0043] 2) Synthesis of COF3@ZIF-67
[0044] Dissolve 50 mg of ZIF-67 in a solution of terephthalaldehyde (0.02 mmol) in 1,4-dioxane (2 ml) and stir for 1 hour until it becomes uniform, then add tetrakis (4 -Aminophenyl)methane (TAPB, 0.02mmol) and 18 μL of hydrocyanic acid, continued ultrasonic dispersion for 30min. Then heated and stirred at 150 °C for 50 h, centrifuged to separate the product, washed three times with 1,4-dioxane, deionized water and methanol, and dried in a v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com