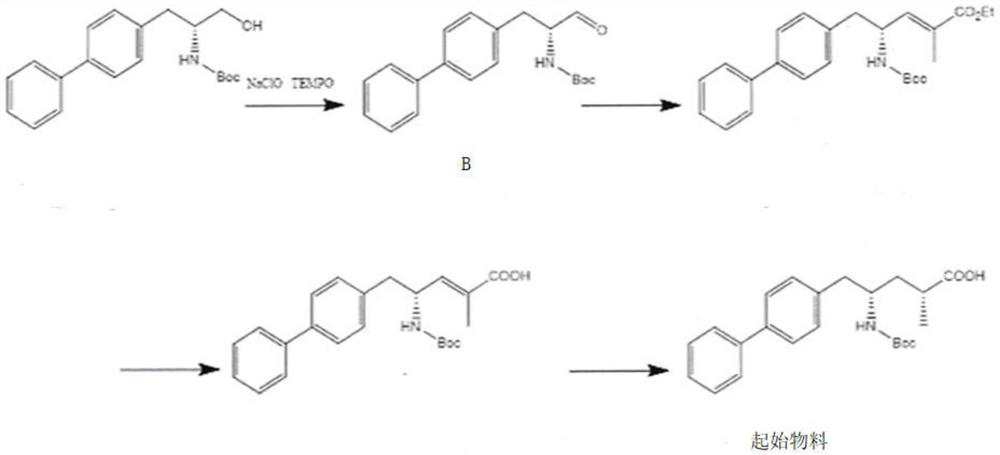
Method for testing genotoxic impurity in sacubitril valsartan sodium starting material
A technology of sacubitrilvaler and sartan sodium, which is applied in the field of analytical chemistry and can solve the problems of inability to complete method verification, sample detection, and low response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Impurity B detection method
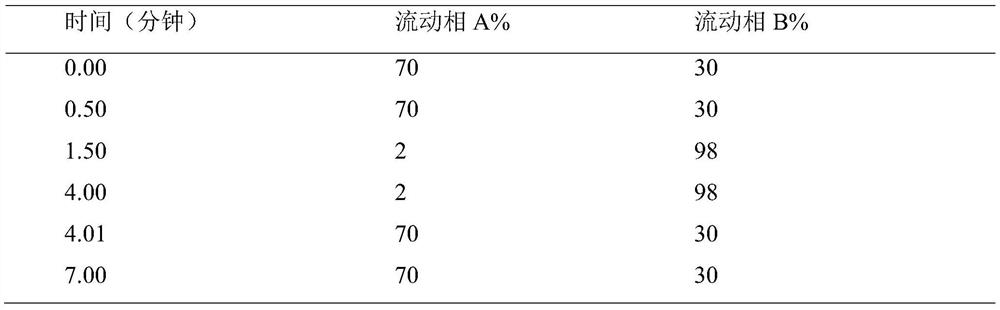
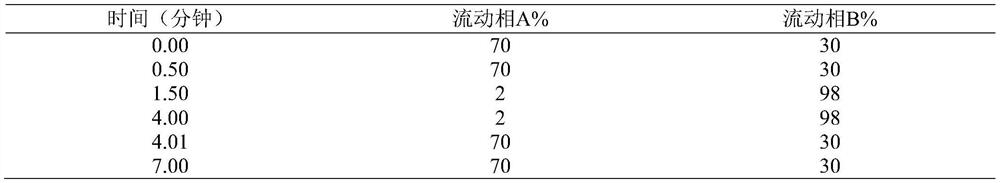
[0038] Take about 0.3g of the test product, weigh it accurately, put it in a 5ml measuring bottle, add 2ml of methanol to dissolve it, add 2ml of 0.1% ammonia water, let it stand for 5 minutes, dilute to the mark with methanol, shake well, filter, and take the subsequent filtrate as the test sample. Test solution: Accurately weigh an appropriate amount of impurity Ⅱa-7 reference substance, add solvent [methanol-0.1% ammonia solution (60:40)] and quantitatively dilute to make a solution containing 210ng of Ⅱa-7 in every 1ml, as the reference substance solution . Measured according to liquid chromatography (Chinese Pharmacopoeia 2015 edition Sibu general rule 0512) and mass spectrometry (Chinese pharmacopoeia 2015 edition Sibu general rule 0431), using octadecylsilane bonded silica gel as filler (InfinityLabPoroshell 120EC-C18, 4.6mm×50mm , 2.7 μm); the mobile phase A is 0.1% ammonia solution, and the mobile phase B is methanol,...
Embodiment 2
[0040] Example 2 Specificity
[0041] Blank (solvent): methanol:0.1% ammonia solution (60:40).
[0042] Reference substance stock solution: Accurately weigh an appropriate amount of impurity B reference substance, add solvent and quantitatively dilute it to make about 2.1 μg of impurity B per 1 ml, as the reference substance solution.
[0043] Reference substance solution: Accurately measure an appropriate amount of the reference substance stock solution, add solvent and quantitatively dilute to make a solution containing about 210ng of impurity B per 1ml, as the reference substance solution.
[0044] The test solution: take about 0.3g of the test sample, weigh it accurately, put it in a 5ml measuring bottle, add 2ml of methanol to dissolve it, add 2ml of 0.1% ammonia water, let it stand for 5 minutes, dilute to the mark with methanol, shake well, filter, Take the continued filtrate as the test solution.
[0045] Mixed solution: Take about 0.3g of the test product, weigh it ...
Embodiment 3
[0050] Embodiment 3 detection limit and quantitative limit
[0051] Quantitation limit solution: Accurately measure the reference substance solution in Example 2, quantitatively dilute step by step with a solvent, accurately measure 3 μl, inject liquid mass spectrometer, record mass spectrogram, and calculate according to the signal-to-noise ratio not lower than 10:1 The limits of quantitation are shown in Table 3.
[0052] Detection limit solution: Accurately measure the reference substance solution in Example 2, quantitatively dilute step by step with a solvent, accurately measure 3 μl, inject the liquid mass spectrometer, record the mass spectrogram, and calculate according to the signal-to-noise ratio not lower than 3:1 The detection limit is shown in Table 2.
[0053] Table 2 Impurity B detection limit and quantitative limit test results
[0054]
[0055] Precisely measure 3 μl of the limit of quantification solution, inject it into the liquid chromatography-mass spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com