Modified antibacterial peptide C-CM8 of Chelonia mydas antibacterial peptide and preparation method and application thereof
A C-CM8, antibacterial peptide technology, applied in the field of biomedicine, can solve problems such as drug resistance of pathogenic microorganisms, and achieve the effect of strong anti-inflammatory activity, small molecular weight, broad-spectrum and high-efficiency antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The chemical synthesis of the modified antimicrobial peptide C-CM8 of the green sea turtle antimicrobial peptide Cm-CATH2 is as follows.
[0022] According to the amino acid sequence and secondary structure of the green sea turtle antimicrobial peptide Cm-CATH2, a fragment of 17 amino acids at the N-terminus was intercepted by using the molecular transformation method of sequence cutting, and the α-helical region of the N-terminus was partially retained, and its C-terminus was completely removed. The modified antimicrobial peptide C-CM5 was designed and obtained without a regular structure, and it was chemically synthesized by the method of peptide solid-phase synthesis. The specific preparation method is as follows:
[0023] 1. According to the amino acid sequence of the modified antimicrobial peptide C-CM8 obtained by design, its complete sequence was synthesized with an automatic peptide synthesizer (433A, Applied Biosystems), and purified by desalting and purifying b...
Embodiment 2
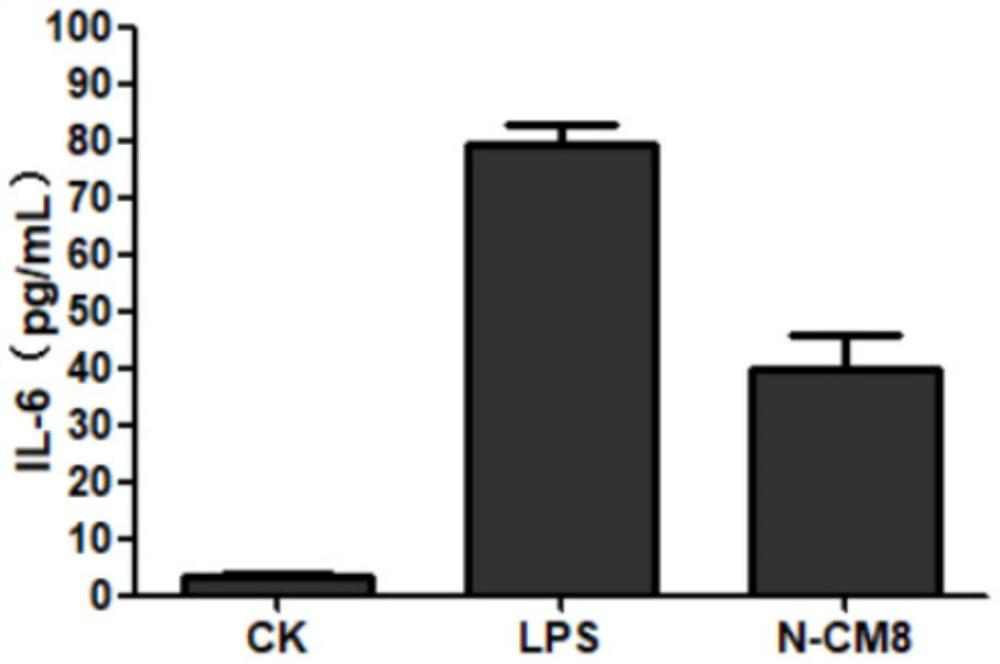
[0030] Determination of antibacterial activity of modified antimicrobial peptide C-CM8:
[0031] Pick the test strains stored on the slant respectively, spread them evenly on the plate of MH solid culture medium (Beijing Suo Laibao Technology Co., Ltd.), place the sterilized 0.5cm diameter filter paper on the surface of the culture medium, add dropwise Dissolve 10 μl of 2 mg / ml antimicrobial peptide C-CM8 sample solution in sterilized deionized water, incubate upside down at 37°C for 18-20 hours, and observe whether the inhibition zone is formed or not.
[0032] If the sample has antibacterial activity, a clear and transparent bacteriostatic zone will be formed around the filter paper, and the larger the bacteriostatic zone, the stronger the antibacterial activity of the sample.
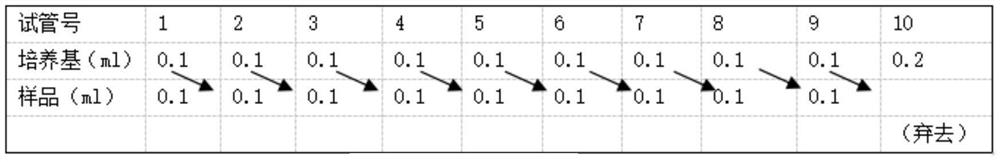
[0033] (2) Determination of Antimicrobial Peptide C-CM8 Minimum Inhibitory Concentration (2-fold dilution method):
[0034] Select the strains with the inhibition zone in the previous experiment to ...
Embodiment 3
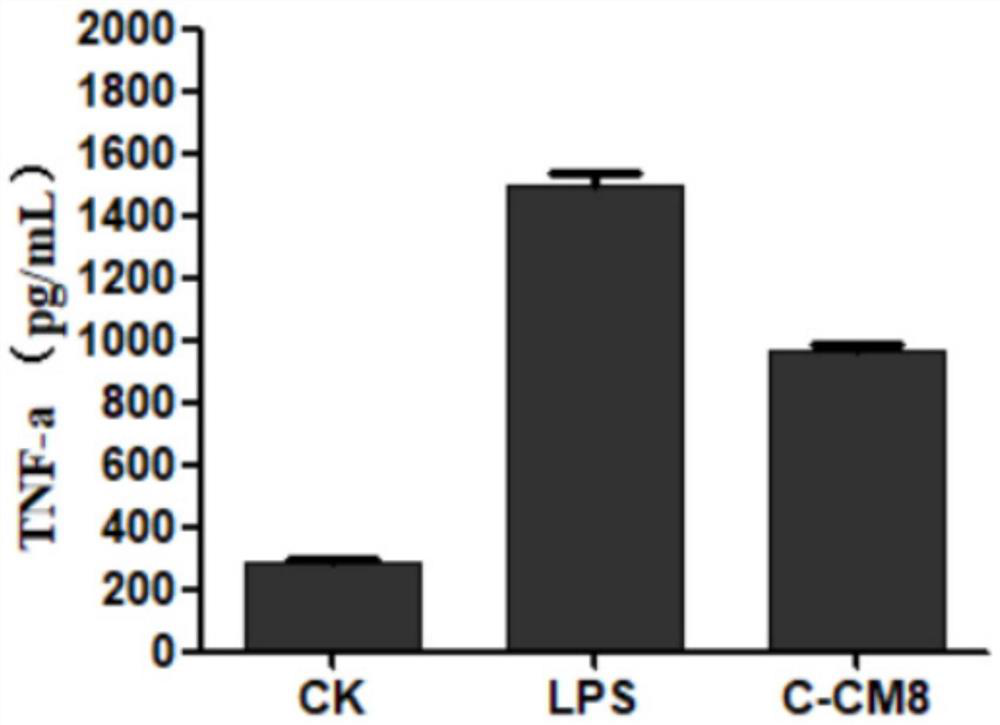
[0044] Determination of hemolytic activity of modified antimicrobial peptide C-CM8:
[0045] The collected rabbit blood was mixed with Alfred's solution for anticoagulation, washed twice with normal saline and resuspended into a suspension of 107-108cell / ml.
[0046] The above-mentioned diluted erythrocyte suspension was mixed with the modified antimicrobial peptide C-CM8 sample dissolved in normal saline, incubated at 37°C for 30 minutes, then centrifuged at 1000rpm for 5 minutes, and the supernatant was measured for absorbance at 540nm. Physiological saline was used as the negative control, 10% Triton X-100 was used as the positive control, and the hemolysis percentage was calculated according to the following formula: hemolysis percentage H%=A sample-A negative control / A positive control×100%.
[0047]The results showed that the sample concentration was 100 μg / ml, and the hemolysis percentage of the modified antimicrobial peptide C-CM8 was 1.34%. It shows that the modified...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com