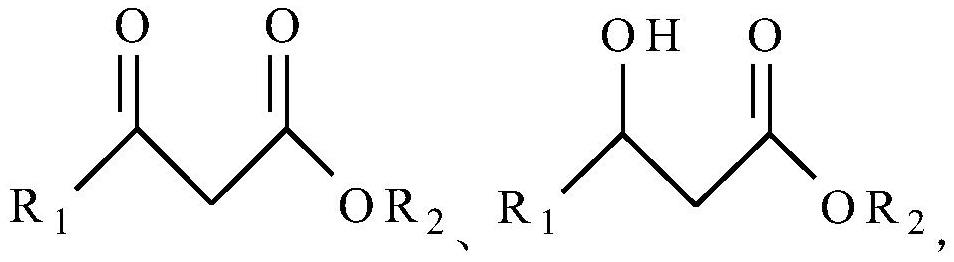Synthesis method of 2-pyrazine carboxylic ester compound
The technology of an ester compound and a synthesis method is applied in the synthesis field of 2-pyrazine carboxylate compound, can solve the problems of complicated reaction process and high cost, and achieves the effects of simple operation, mild reaction conditions and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
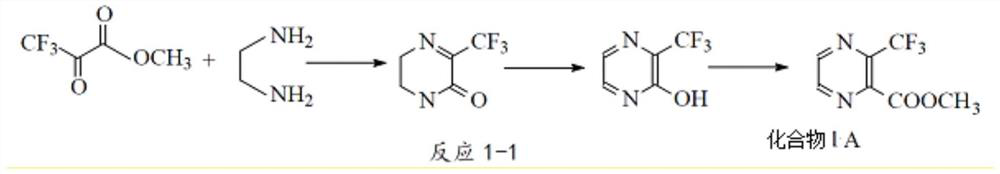
[0021] In a typical embodiment of the present application, a synthetic method of 2-pyrazinecarboxylate compounds is provided, the synthetic method comprising: step S1, compound 1 and glyoxal dioxime in a Lewis acid catalyst Under the action of addition reaction, intermediate 1 is obtained; step S2, intermediate 1 undergoes the first dehydration reaction to obtain 2-pyrazinecarboxylate compounds, wherein, compound 1, intermediate 1, 2-pyrazinecarboxylate The general structural formula of ester compounds is as follows:
[0022] R 1 for C 1 ~C 15 Substituted or unsubstituted alkyl, R 2 for C 1 ~C 10 of alkyl.
[0023] The preparation cost (or commercial price) of the starting material compound 1 that the present invention adopts is generally far lower than trifluoropyruvate methyl ester compound; The carbon-nitrogen double bond is added to reduce the probability of self-polymerization of compound 1 as much as possible, so as to obtain a ring-forming intermediate, and the...
Embodiment 1
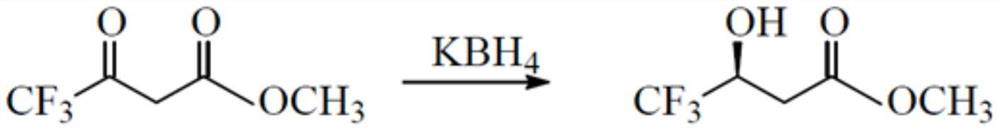
[0039] Step 1: Restore
[0040]
[0041] First, add 150mL of tetrahydrofuran and 20.9g (0.387mol) of potassium borohydride to a 500mL glass three-neck flask, under nitrogen protection, stir and raise the temperature to 40-45°C, control the temperature between 40-45°C, drop Add 50g (0.352mol) of a mixture of methyl trifluoroacetoacetate and 50mL of tetrahydrofuran, and finish adding in about 1 hour. After the addition, raise the temperature to 60-65°C, continue to keep warm for 4 hours, take samples, and after the central control is qualified, Cool down to 20-25°C and wait for hydrolysis; in another 1000mL reaction bottle, add 209g of water and 91g of 31% hydrochloric acid (0.774mol), replace with nitrogen 3-5 times, and stir to cool down to 0-10°C. Slowly add the aforementioned reaction liquid into hydrochloric acid water for hydrolysis; while adding, continue to stir and lower the temperature, and control the adding speed so that the hydrolysis temperature is kept between ...
Embodiment 2
[0055] The difference between Example 2 and Example 1 is that the catalyst for the fourth step reaction is 0.80 g of zinc chloride, and the temperature of the addition reaction is 90° C. to finally obtain methyl 3-trifluoromethyl-2-pyrazinecarboxylate .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com