Gene deletion type klebsiella pneumoniae attenuated live vaccine, preparation method and application
A Klebsiella pneumoniae and attenuated live vaccine technology, applied in the field of bioengineering, can solve the problems of limited protection range, complicated production process, and low protection power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Such as figure 1 As shown, the preparation method of the gene-deleted Klebsiella pneumoniae live attenuated vaccine provided by the embodiments of the present invention comprises the following steps:
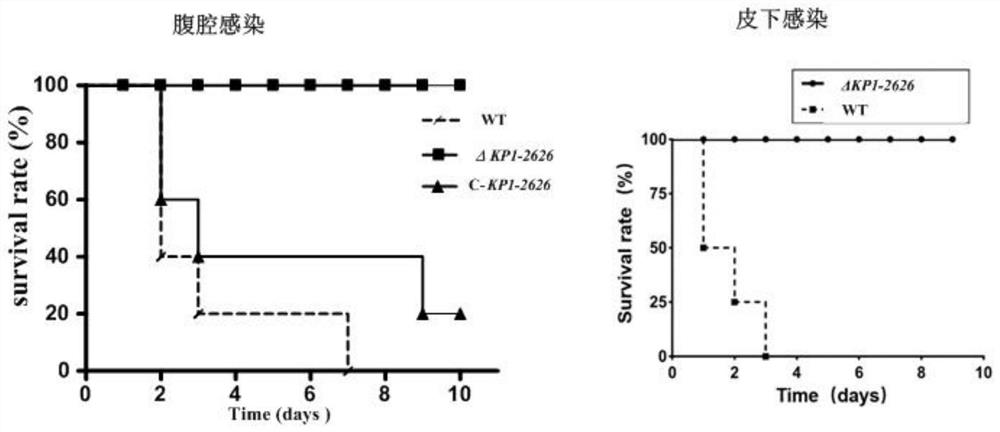
[0040]S101, preparing a virulent Klebsiella pneumoniae NTUH-K2044 strain with a KP1_RS12260 gene deletion strain, and analyzing the pathogenicity of Klebsiella pneumoniae KP1_RS12260 gene deletion strains infecting mice through different routes;
[0041] S102, select the virulent Klebsiella pneumoniae NTUH-K2044 strain KP1_RS12260 gene deletion strain, and use LB medium to culture and proliferate overnight on a bacterial shaker at 37°C and 220r / min;
[0042] S103, on the next day, dilute it 1:100 into fresh LB culture medium and cultivate to OD600=1.4, centrifuge for 10 minutes to collect the centrifuged sediment, wash the bacteria twice with normal saline, and then resuspend with normal saline until the bacterial concentration reaches 10 5 CFU / ml;
[0043] S104, Effic...
Embodiment 1
[0046] Example 1: Preparation and evaluation of Klebsiella pneumoniae KP1_RS12260 gene deletion strain of the present invention.
[0047] 1. Construction of Klebsiella pneumoniae KP1_RS12260 gene deletion strain
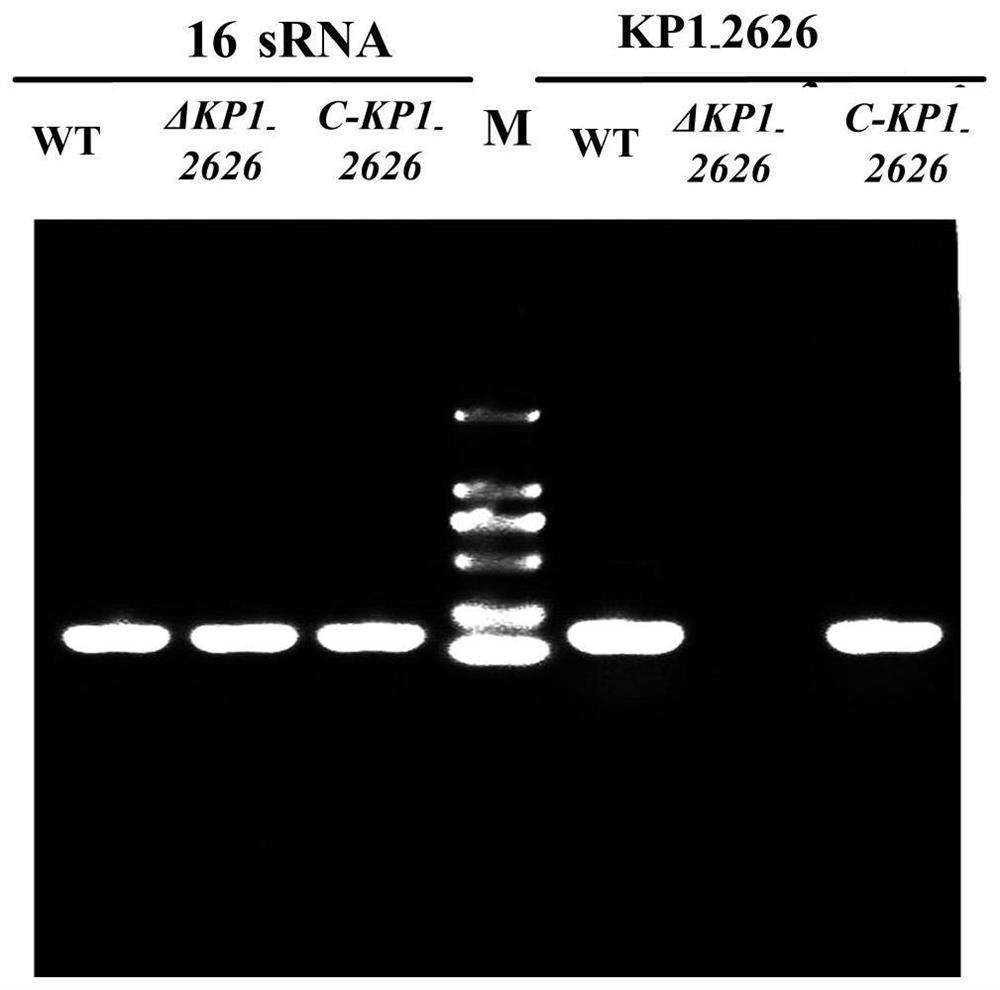
[0048] A 1669bp gene fragment of the upper and lower flanking sequences of the Klebsiella pneumoniae KP1_RS12260 gene was obtained by fusion PCR. Cloned to the temperature-sensitive suicide carrier pKO3-Km to obtain the recombinant mutant cassette plasmid, and electroporated into the Klebsiella pneumoniae wild strain (WT strain) NTUH-K2044 to obtain the KP1_RS12260 gene deletion strain (ΔKP1_RS12260). Then the 1876bp fragment comprising the KP1_RS12260 gene coding region, the promoter binding region and the transcription termination region was cloned onto the pGEM-T-easy vector and transformed into the KP1_RS12260 gene deletion strain to obtain the complementing strain (C-KP1_RS12260) (see figure 2 ).
[0049] The gene sequence corresponding to the attenuated vacc...
Embodiment 2
[0060] Embodiment 2: preparation and efficacy test of Klebsiella pneumoniae KP1_RS12260 gene deletion live attenuated vaccine of the present invention.
[0061] Select the KP1_RS12260 gene deletion strain of the virulent Klebsiella pneumoniae NTUH-K2044 strain, use LB medium to culture and proliferate on a bacterial shaker at 37°C 220r / min overnight, and dilute it into fresh LB culture medium at 1:100 the next day Cultivate to about OD600=1.4, centrifuge for 10 minutes to collect the centrifuged sediment, wash the bacteria twice with normal saline, and then resuspend with normal saline until the bacterial concentration reaches about 10 5 CFU / ml, take 100ul resuspended bacteria solution and inject subcutaneously to immunize BALB / C mice, and the control group is injected with the same amount of PBS. Fourteen days after the initial infection, the same amount of bacteria was used to boost the immunization once again. Blood was collected from the tail vein on day 28 of the initial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com