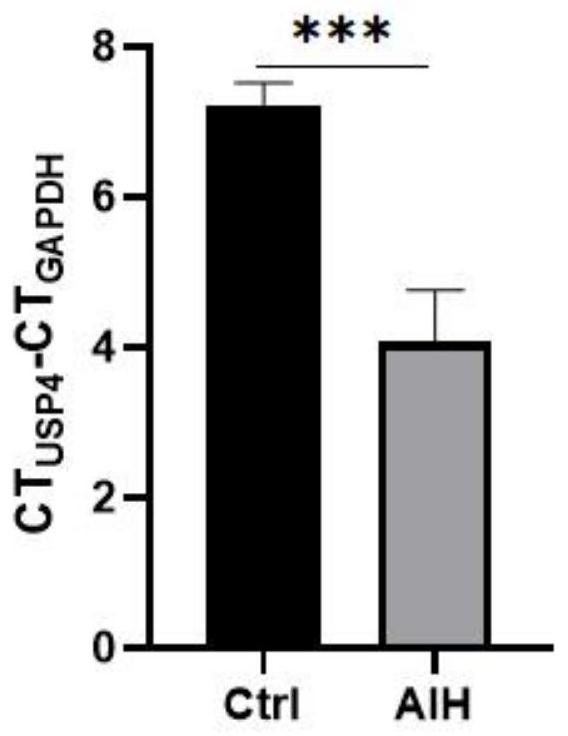
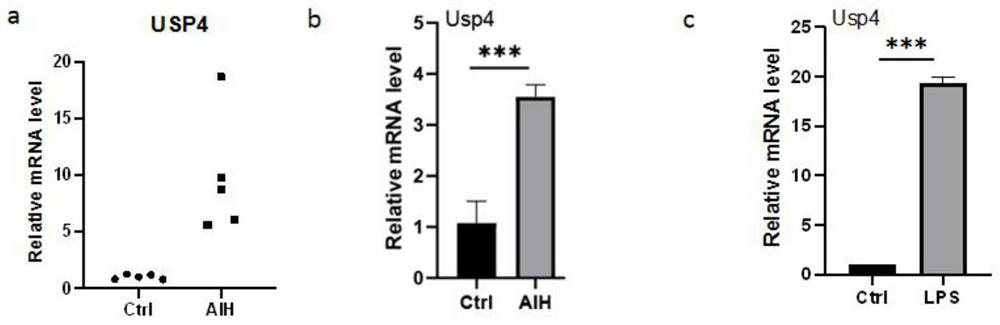
Application of USP4 as autoimmune liver disease biomarker
A technology of autoimmunity and markers, applied in the field of medical testing, can solve problems such as limited means and unclear results, and achieve the effect of obvious technical effect and important clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] 3.2 Preparation of peripheral blood mononuclear cells
[0043] (1) Take fresh anticoagulated blood from healthy subjects or patients with autoimmune liver disease described in step 3.1, centrifuge at 3000rpm for 10min, and keep the supernatant.
[0044] (2) Absorb the supernatant, that is, the plasma, with a rubber-tipped dropper.
[0045](3) Transfer the remaining peripheral blood after removing plasma to a 15ml centrifuge tube, add PBS for dilution, and the ratio of peripheral blood to diluent is 1:2.
[0046] (4) Add Ficoll to the 50ml centrifuge tube, slowly add the peripheral blood diluted in step (3) along the inclined tube wall, the ratio of Ficoll to diluted blood is 1:1.
[0047] (5) 20°C, 1800rpm (500-1100g), centrifuge for 30min.
[0048] (6) After centrifugation, the centrifuge tube is divided into four layers from top to bottom. The first layer is the plasma layer, the second layer is the ring-shaped milky white lymphocyte layer, the third layer is the t...
Embodiment 1
[0053] Embodiment 1 is used to detect the kit of USP4 mRNA
[0054] The kit is used for the detection of the relative expression of USP4 gene for clinical testing purposes. Real-time fluorescent quantitative PCR technology is used to measure the expression level of USP4 gene in peripheral blood mononuclear cells of patients with liver disease. At the same time, it is helpful for the diagnosis of autoimmune liver disease.
[0055] Kit components include:
[0056] (1) Sample total RNA extraction reagents: TRIZOL, chloroform, ethanol, DEPC water;
[0057] (2) Total RNA reverse transcription reagent: Thermo Fisher's Revert First Strand cDNA Synthesis Kit.
[0058] (3) Real-time fluorescent quantitative PCR reaction reagent: upstream and downstream standard primers of USP4 and GAPDH, SYBR Green (Thermo Fisher). The primer sequences are shown in Table 1 below,
[0059] Table 1
[0060]
Embodiment 2
[0061] Embodiment 2 Real-time fluorescence quantitative PCR detects the method for the mRNA level of USP4
[0062] 1. RNA extraction
[0063] ①Take 50 mg of mouse liver tissue, healthy subjects or human peripheral blood mononuclear cells from patients with autoimmune liver disease prepared in sample preparation, add 1 ml of Trizol, grind / pip and beat sufficiently, and let stand at room temperature for 5 minutes to completely separate the nuclei Protein complex; for AML12 hepatocytes cultured in six-well plates, discard the medium and wash with PBS for 1 to 2 times, then add 1ml of Trizol to the wells, pipette fully and let stand at room temperature for 5 minutes to completely separate nucleoproteins Complex;
[0064] ②Add 0.2ml chloroform respectively, shake vigorously by hand for 15 seconds, wait at room temperature for 2 minutes, centrifuge at 4°C and 13000rpm for 15 minutes, it can be seen that the solution is divided into three layers, from top to bottom are transparent w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com