Iron phosphate preparation method
A technology of ferric phosphate and ferrous phosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as difficulty in meeting the requirements of battery-grade and food-grade ferric phosphate, low purity of ferric phosphate, etc., and reduce environmental pollution. The effect of carrying pressure, avoiding iron-containing waste residue, and ensuring purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
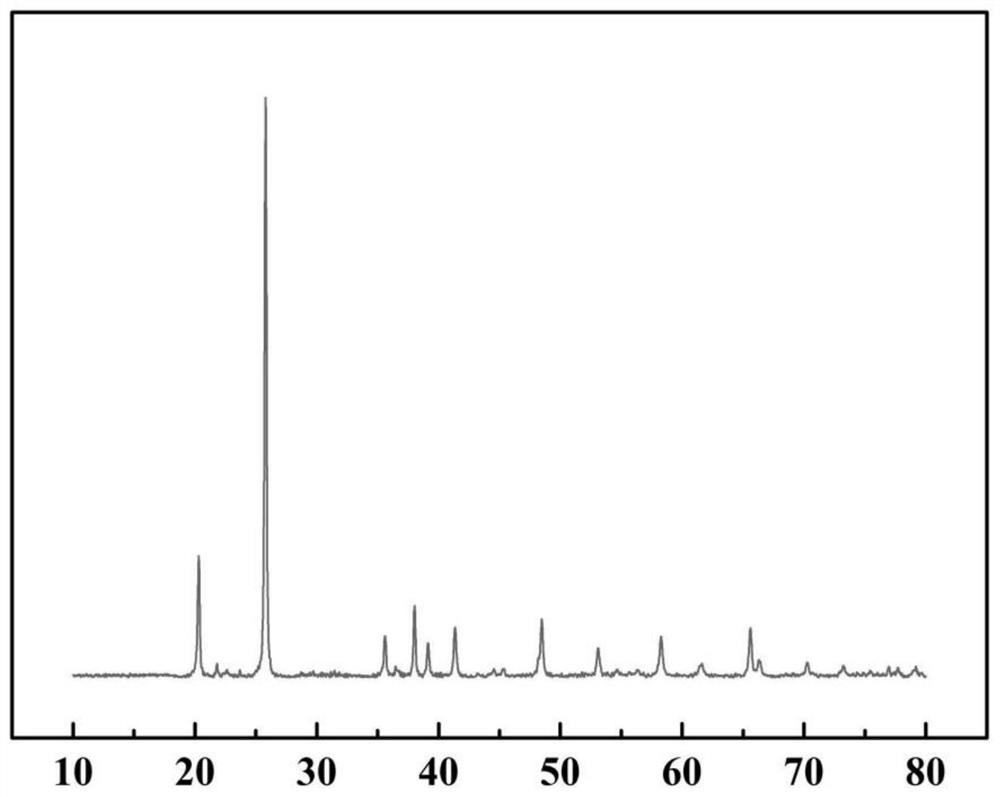
[0034] Please combine figure 1 , figure 2 , the present embodiment provides a method for preparing ferric phosphate, comprising the steps of:
[0035] Take 120 mL of ferrous sulfate solution with a molar concentration of 1.3 mol / L, add it to 80 mL of diammonium hydrogen phosphate solution with a molar concentration of 1.3 mol / L, stir and add NH 3 ·H 2 O, adjust the pH value to 7, and after 60 minutes of reaction, ferrous phosphate precipitation is obtained.
[0036] After filtering and washing, transfer ferrous phosphate to a beaker, add 100g of water for beating, and then add 1.3mol / L of FeSO 4 Solution 24mL and 0.05g dithiocarbamate capture agent, add 0.4mol phosphoric acid to the residue after filtration, add H 2 o 2 About 3.35g, with NH 3 -H 2 O (1:3) adjusted the pH of the system to 2.0, raised the temperature to 95°C for 180 minutes, filtered and dried the precipitate to obtain FePO4·2H2O (ferric phosphate dihydrate) powder.
[0037] Weigh FePO4·2H2O powder to a...
Embodiment 2
[0041] Add 420 mL of ferrous sulfate solution with a molar concentration of 0.5 mol / L to 140 mL of diammonium hydrogen phosphate solution with a molar concentration of 1.0 mol / L, add 0.2 g of dispersant CTAB to the solution, and add 32% NaOH to adjust When the pH value reaches 5.0, filter and wash after reacting for 20 minutes to obtain ferrous phosphate precipitate;
[0042]Add ferrous phosphate to 40% of the solid content and add water for beating, then add 20g of sulfuric acid, 5g of sodium sulfate and 1g of xanthate capture agent, add 0.5mol of phosphoric acid, filter after 0.5 hours of reaction, and then pour into the residue Add H to 2 o 2 About 4.15g and use 16% NaOH to adjust the pH of the system to 1.5. After 2 hours of reaction, raise the temperature to 80 degrees and age for 6 hours to obtain FePO 4 .2H 2 O (ferric phosphate dihydrate) powder.
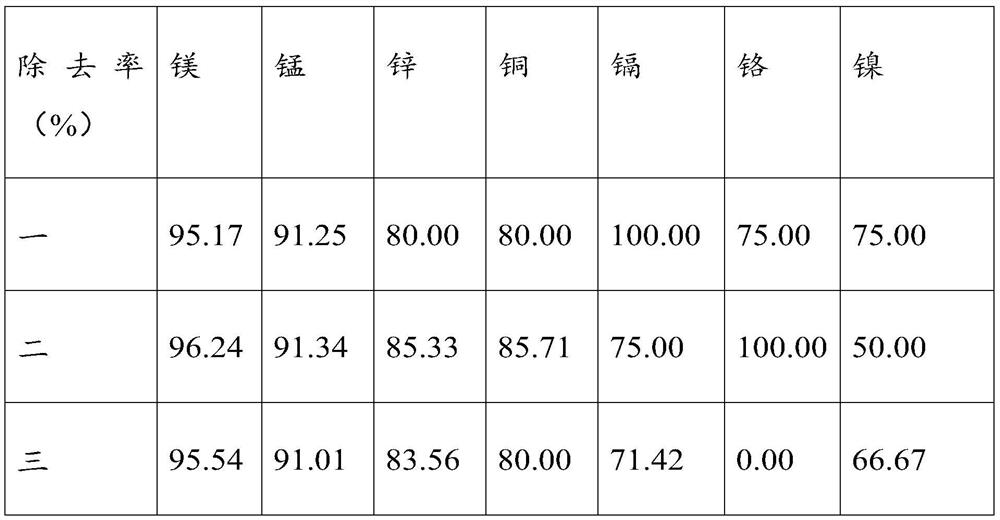
[0043] Weigh FePO4·2H2O powder to analyze its purity, the purity of ferric phosphate dihydrate is 99.6%, and the conte...
Embodiment 3
[0047] 40 mL of diammonium hydrogen phosphate (NH 4 h 2 PO 4 ), added to 120mL molar concentration of 1.3mol / L ferrous sulfate (FeSO 4 ) solution, ammonia monohydrate (NH 3 .H 2 (0), adjust its pH value to 4.0, after reacting for 10min, filter and wash to obtain ferrous phosphate precipitation.
[0048] After ferrous phosphate was beaten with water according to 30% solid content, 5 g of hydrochloric acid, 10 g of ammonium sulfate and 0.05 g of octahydroxyquinoline were added, and then 0.5 mol of phosphoric acid and 0.04 mol of ammonium dihydrogen phosphate were added. The reaction was filtered for 0.5 h, and H was added to the residue 2 o 2 A total of 3.28g, with NH 3 ·H 2 O to adjust the pH of the system to 3.0, after aging for 40 minutes, raise the temperature to 90°C for 3 hours, filter and dry to obtain FePO 4 .2H 2 O powder. .
[0049] Weigh FePO4 2H2O powder to analyze its purity, the purity of ferric phosphate dihydrate is 99.5%, and the content of impurity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com