Solid preparation containing composite-form telmisartan and preparation method of solid preparation
A technology for telmisartan and solid preparation, which is applied to the composite form of telmisartan solid preparation with low content of alkaline substances and the field of preparation thereof, and can solve the problems of poor dissolution rate, high content of alkaline substances, slow dissolution rate, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
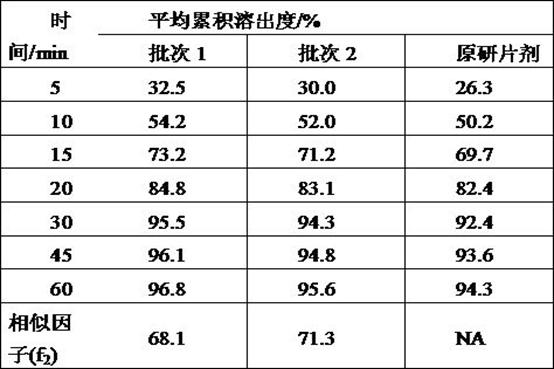
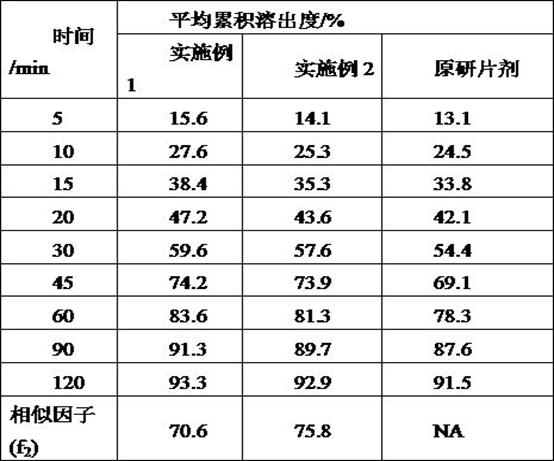
Examples
preparation example 1
[0096]Preparation 1: Preparation of high-uniform small particulate Misassartan crystals
[0097]S1: Preparation of high-uniform small particulate Misassartan crystals
[0098]1) In the reaction vessel with reflux condensation, the metamin (purity greater than 99%) is dissolved in formic acid-isopropanol (volume ratio 1: 5), formulated into a concentration of 8 g / 100 ml For Misassarta solution A, heated and refluxed to completely dissolved, cooling to about 75 ° C, spare; simultaneously, the deionized water is cooled to 1-2 ° C, as solvent B;
[0099]2) In the tubular reactor connected to a crystal growth, the above-described Timartan solution A and the solvent B are pumped into the reactor in the reactor via the top and side of the two different inlets, and generate crystals. Nucleic; wherein the solution A flow rate is 10 ml / sec, the solvent B flow is 60 ml / sec; the formed mixture of crystal microparticles is incorporated into the crystal kettle;
[0100]3) In a crystal tank having a tem...
preparation example 2
[0101]Preparation 2: Making replacement Misassarta micronized solid dispersion
[0102]S1: Making replacement Misassartan - cycloderathry premix
[0103]1) We will weigh the above-mentioned small particles Timartan 0.1 kg and 0.5 kg of composite cycloderathry (from 80% by weight of sulfury ether-β-cyclodextrin and 20% by weight of sulfury ether-γ). Cyclodextrin composition), a total of about 0.6 kg; a mixed solvent (ethanol volume ratio of ethanol) 4.5 L is pre-added in the water bath reaction kettle, and the proposed composite cyclodextrial derivative is added to the reactor, Heat until 70 ~ 72 ° C, stirred to dissolution, and then added to the Misassartan continued to stir for 15 min for sufficient mixing; stop heating and stirring, cooled to room temperature;
[0104]2) The above-mentioned solution is sprayed and dried to obtain gray-white fine particles; the spray pressure is about 3 bar, and the outlet air temperature is about 80 ° C. The resulting dry particle powder was over 140 mesh ...
preparation example 3A
[0107]Preparation Example 3A: Making replacement Misaartan Compound Particles 1
[0108]1) Make a crude rice salt
[0109]At room temperature, 86 g of sodium hydroxide was dissolved in 1.2 kg of deionized water to obtain an alkaline solution, stirred sufficiently to dissolve it; add 1 kg of Misassartan, heating to 35 ° C for 20 min; then cooled to room temperature; stirring The product mixture was slowly added to about 10 l ethanol, stirred slowly at 4 ° C for 1 h, and then filtered after 30 min, and the resulting filter cake was dried to give a crude material.
[0110]S2: Making replacement Misassart Sodium composite particles
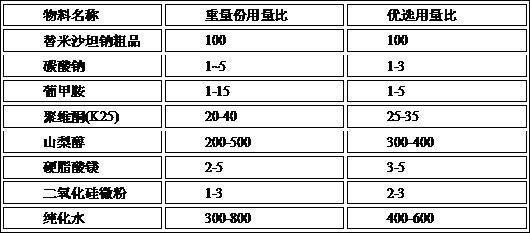
[0111]1) Under room temperature and rapid stirring conditions, 1 kg of misa sodium and 10.5 g of sodium carbonate, 40 g of gluchanamine, 320 g Piwifen is added to 5L deionized water, and the material is stirred until it is completely clarified to obtain it. Mishaan sodium premix solution;
[0112]2) The sorbitol will be proud of the sorbitol, first adding the half amount ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com