Preparation method of triazine ring
A technology of triazine ring and ring closure reaction, which is applied in the field of medicine, can solve the problems of unavailable input, prominent environmental protection problems, complex solvents, etc., and achieve the effects of increased product purity, reduced environmental protection costs, and simple treatment of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
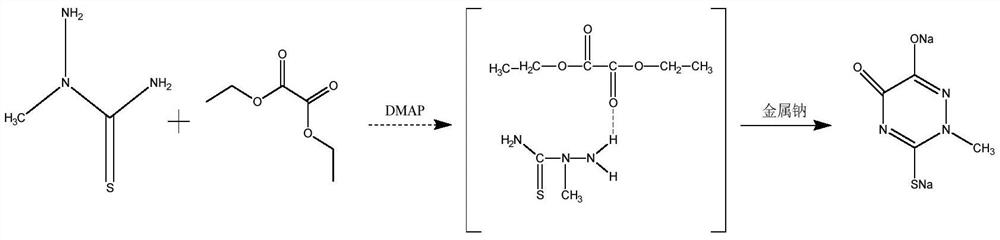
[0051] (1) Add 24.2g of 2-methylthiosemicarbazide, 70.5g of diethyl oxalate, and 20g of DMAP into a 500mL three-necked flask with an exhaust gas absorption device, raise the temperature to 50°C, reflux for 1 hour, start to slowly add 12g of sodium metal, continue The reaction was refluxed for 3 hours.
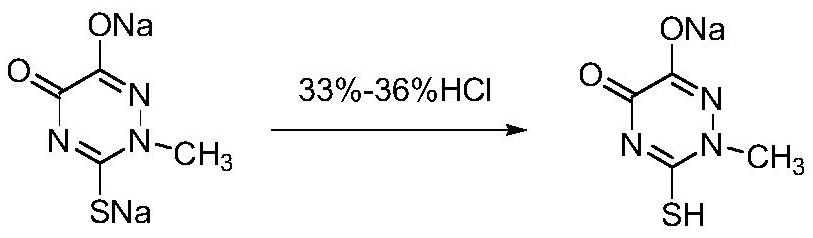
[0052] (2) After the cyclization reaction is completed, add 35% hydrochloric acid to remove excess DMAP and sodium ethoxide, control the pH value to 6.7, cool down and filter to obtain 67.5 g of a wet product of triazine ring sodium salt.
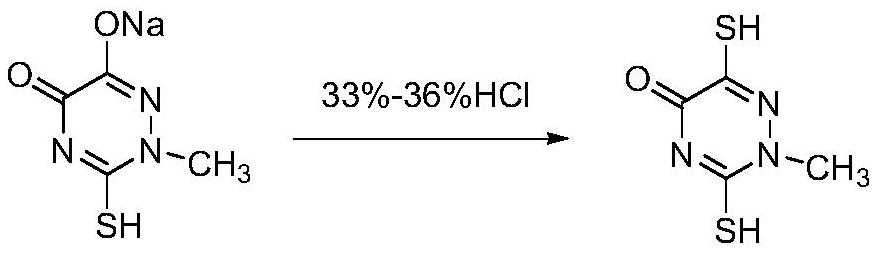
[0053] (3) Add 67.5g of triazine ring sodium salt wet product and 135g of purified water to a 250mL three-necked flask and heat up to 65°C, add 35% hydrochloric acid to adjust the pH value to 1.5, add seed crystals and cool to 10°C for crystallization for 1h, filter , and dried to obtain 32.9 g of the triazine ring product, with a yield of 90.1%, a liquid phase purity of 99.8%, and a mass content of 99.0%.
[0054] After testing, the triazi...
Embodiment 2
[0059] (1) Add 24.2g of 2-methylthiosemicarbazide, 84g of diethyl oxalate and 18g of DMAP into a 500mL three-necked flask equipped with an exhaust gas absorption device, raise the temperature to 60°C, reflux for 1 hour, start slowly adding 13g of sodium metal, and continue to reflux React for 2 hours.
[0060](2) After the cyclization reaction, add 33% hydrochloric acid to remove excess DMAP and sodium ethoxide, control the pH value to 7.5, cool down and filter to obtain 63.6 g of a wet product of triazine ring sodium salt.
[0061] (3) Add 63.6g of triazine ring sodium salt wet product and 127.2g of purified water into a 250mL three-necked flask and raise the temperature to 75°C, add 33% hydrochloric acid to adjust the pH value to 2.5, add seed crystals and cool to 15°C for crystallization for 2 hours, Filtration and drying yielded 32.5 g of the triazine ring product, with a yield of 89.0%, a liquid phase purity of 99.8%, and a mass content of 99.2%.
Embodiment 3
[0063] (1) Add 24.2g of 2-methylthiosemicarbazide, 84g of diethyl oxalate, and 18g of DMAP into a 500mL three-neck flask equipped with an exhaust gas absorption device, raise the temperature to 55°C, reflux for 1 hour, start to slowly add 13g of sodium metal, and continue to reflux React for 2.5 hours.
[0064] (2) After the cyclization reaction is completed, add 36% hydrochloric acid to remove excess DMAP and sodium ethoxide, control the pH value to 7.0, cool down and filter to obtain 65.4 g of a wet product of triazine ring sodium salt.
[0065] (3) Add 65.4g of triazine ring sodium salt wet product and 130.8g of purified water to a 250mL three-necked flask and raise the temperature to 70°C, add 36% hydrochloric acid to adjust the pH value to 2.0, add seed crystals and cool to 12°C for crystallization for 1.5h , filtered, and dried to obtain 33.0 g of the triazine ring product, with a yield of 90.4%, a liquid phase purity of 99.7%, and a mass content of 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com