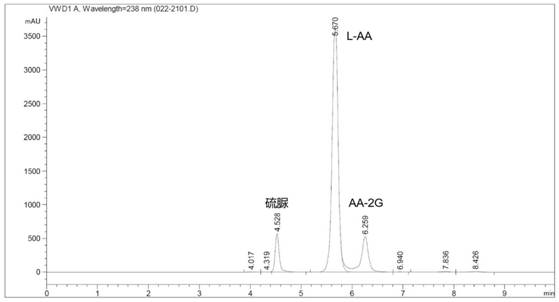
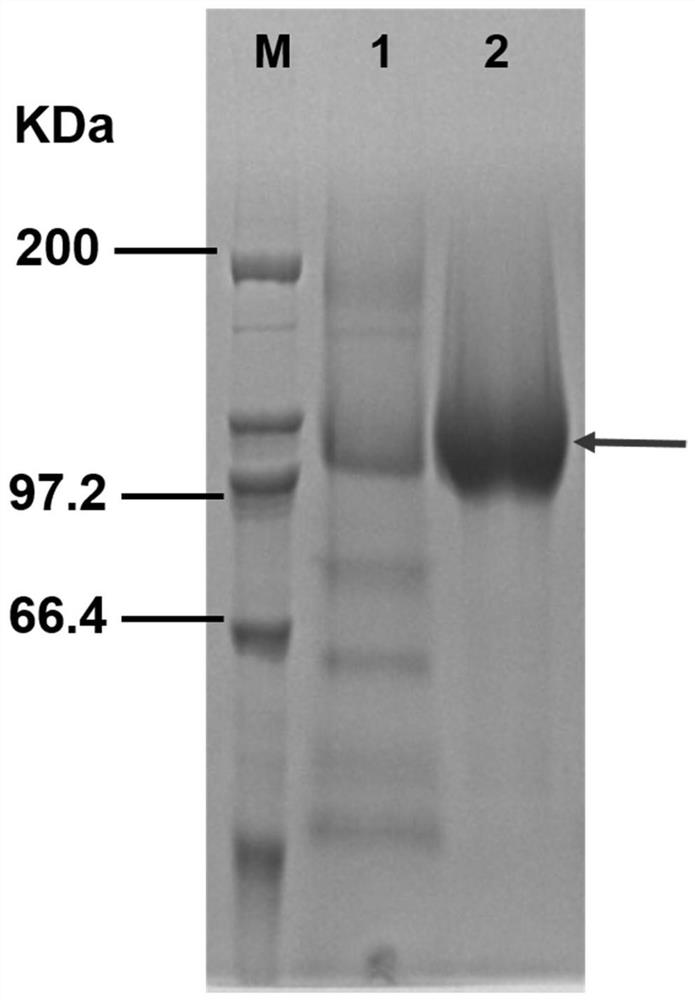
Alpha-glycosidase gene mutant and application thereof in preparation of 2-O-alpha-D-glucosyl-L-ascorbic acid
A technology of ascorbic acid and 2-O-, which is applied in the field of α-glucosidase mutants, can solve the problems of less research and achieve the effect of improving enzyme activity, improving conversion rate and product quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
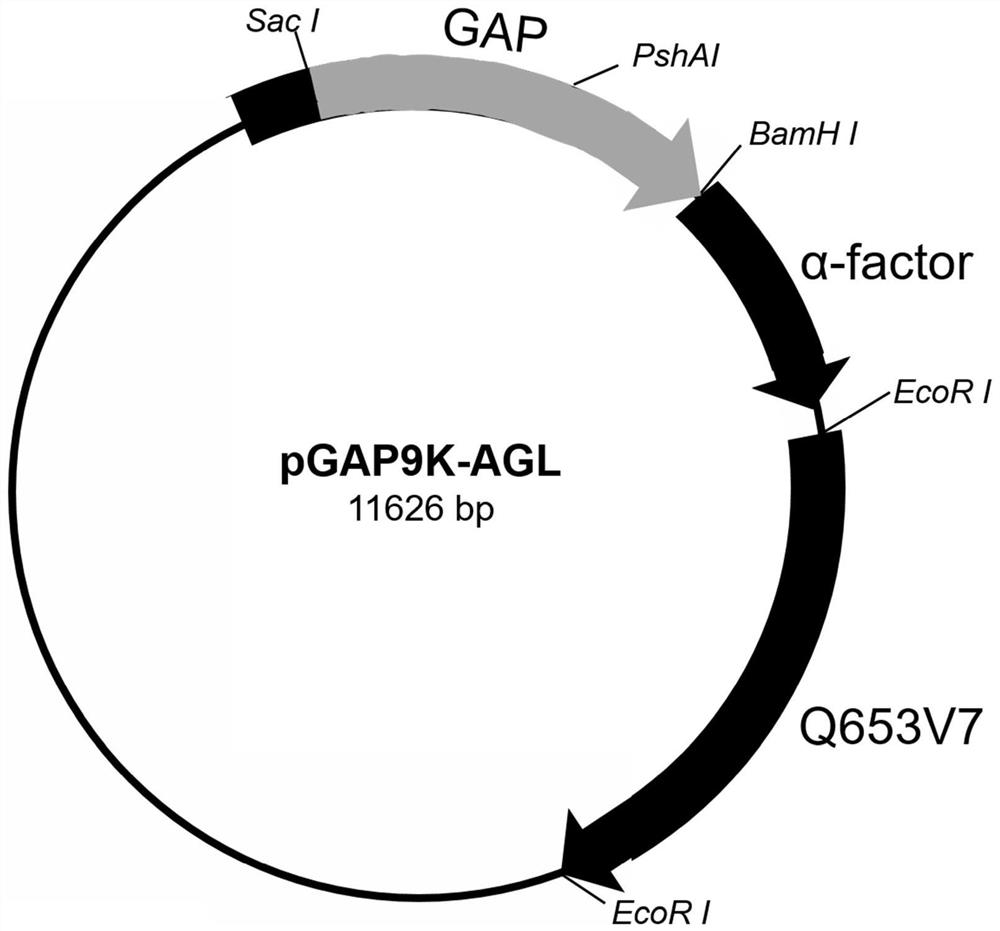
[0035] Example 1: Construction of recombinant vector (pGAP9K-AGL)
[0036] from Oryza sativa subsp. japonica (Rice) The α-glucosidase gene, whose nucleotide sequence is shown in SEQ ID NO.2, was synthesized by Nanjing GenScript Technology Co., Ltd. (GenScript) and then cloned into the restriction endonucleic acid of the eukaryotic expression vector pGAP9K enzyme site EcoRI After that, and add a 6-his tag to its N-terminus, the recombinant vector pGAP9K-AGL (refer to figure 1 ).
Embodiment 2
[0037] Embodiment 2: Preparation of α-glucosidase mutant
[0038] According to the nucleotide sequence of α-glucosidase as shown in sequence SEQ NO.2, primers introducing Y270F, W373L, M411F, R491K and W504L mutations were designed and synthesized, and site-directed mutagenesis kit (Vazyme. Co.) was used to The obtained recombinant plasmid pGAP9K-AGL of embodiment 1 is template, through PCR amplification, product DpnI Digestion, recombinant transformation, plasmid extraction, sequencing verification and other steps obtained the mutant recombinant plasmids pGAP9K-AGL-Y270F, pGAP9K-AGL-W373L, pGAP9K-AGL-M411F, pGAP9K-AGL-R491K and pGAP9K mutated at the corresponding sites -AGL-W504L.
[0039] The site-directed mutagenesis primers for introducing the Y270F mutation are (underlined are mutated bases):
[0040] Forward primer: SEQID NO.8 5’ TGTAGA TTC GGTTATAAGAACGTTGCTGATT 3’
[0041] Reverse primer: SEQID NO.9 5'ATAACC GAA TCTACATTGGTGAAAACCGAA 3’
[0042] The site-direc...
Embodiment 3
[0055] Embodiment 3: Shake flask fermentation produces enzyme
[0056] The recombinant Pichia strains Y270F, W373L, M411F, R491K and W504L obtained in Example 2 were inoculated in YPD medium respectively, and after being cultivated at 30° C. for 24 h, they were transferred to 50 mL of BMGY medium with 10% inoculum size. Cultivate in a shaker at 30°C and 200rpm for 18 hours, then centrifuge at 4000rpm for 5min, discard the supernatant, collect the bacteria, hang the bacteria with 200 mL of BMGY medium, and culture in a constant temperature shaker at 30°C for 72h, supplemented every 12h Supplement the final concentration of 1% glucose as carbon source. After the shake flask fermentation is finished, centrifuge at 5000rpm for 20min, and the supernatant is the crude enzyme solution of the desired α-glucosidase mutants Y270F, W373L, M411F, R491K and W504L. The recombinant Pichia pastoris strains with higher protein expression and higher enzyme activity were selected for continuous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com