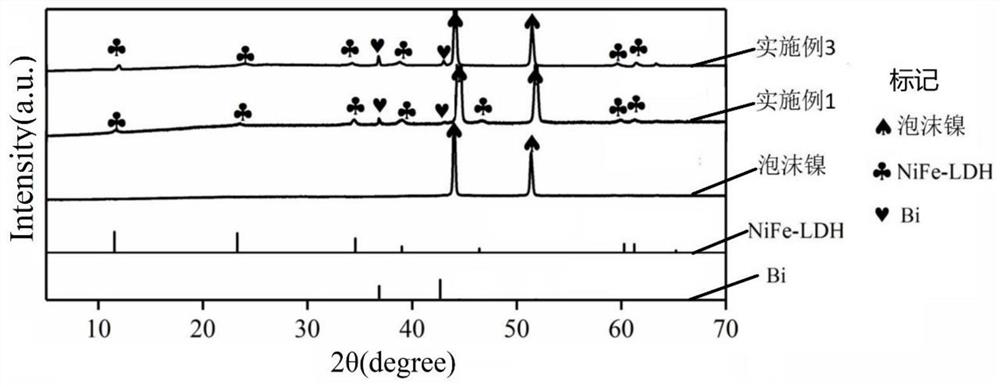
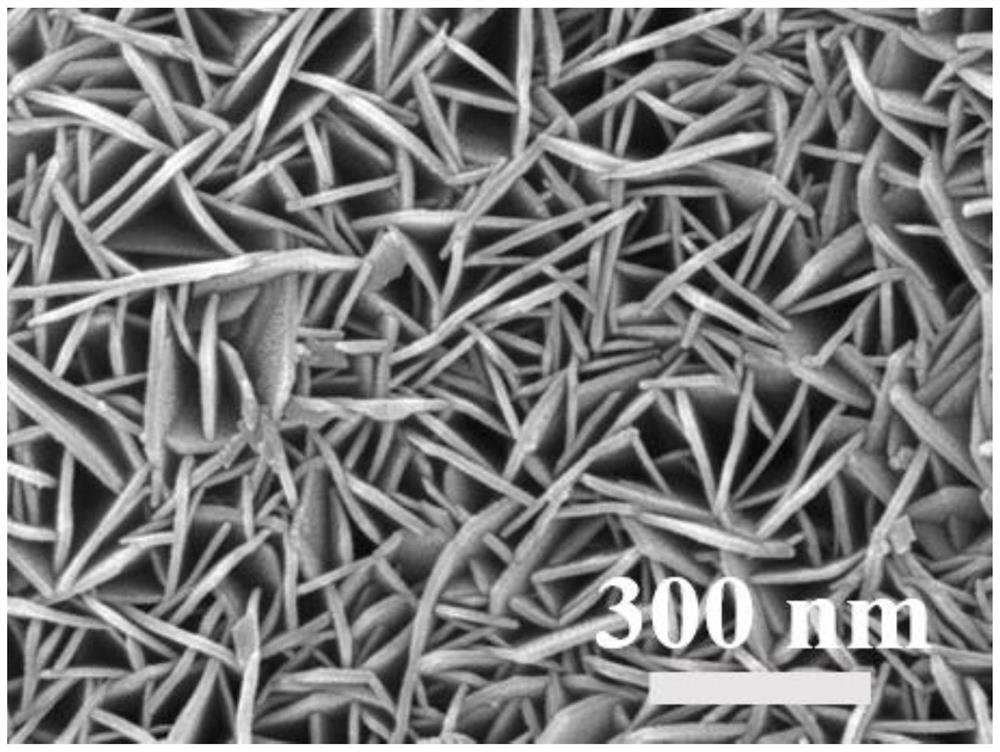
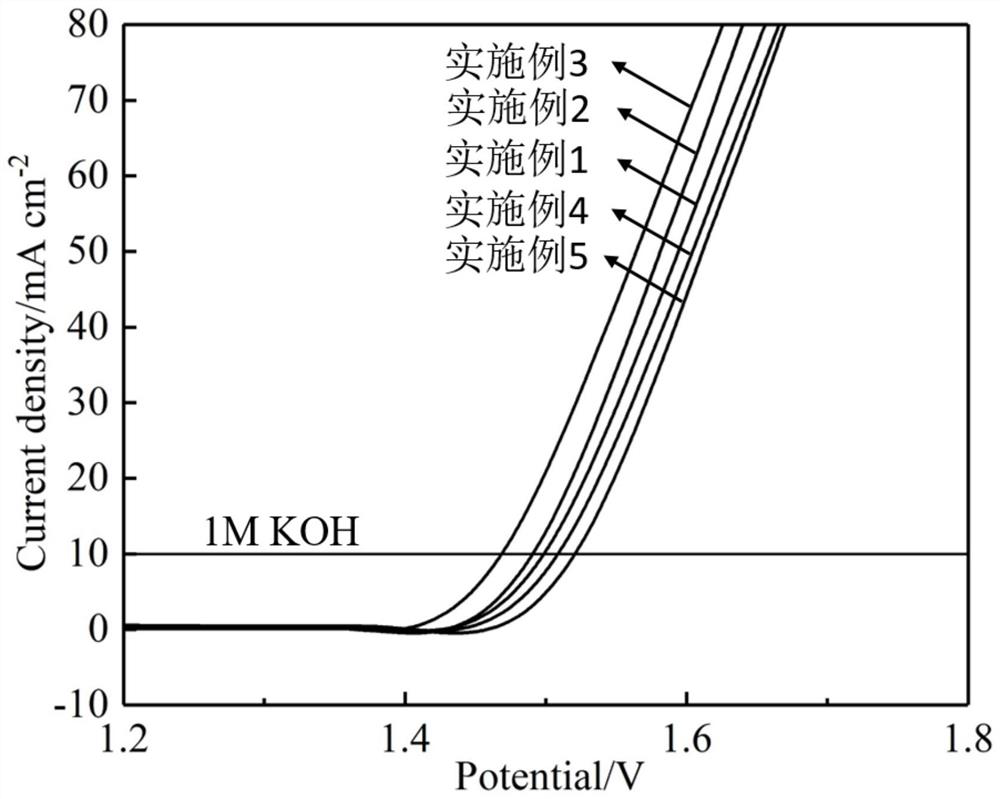
Preparation method of Bi@NiFe-LDH/NF composite material
A composite material, 2·6H2O technology, applied in the field of electrocatalysis, can solve the problems of small Tafel slope, low potential and high cost, and achieve the effects of uniform product, high yield and fast heating speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Put nickel foam (10mm×13mm×0.3mm) into 37wt% hydrochloric acid solution and ultrasonicate for 4 minutes to remove the NiO layer on the surface, then ultrasonically in deionized water and absolute ethanol for 8 minutes each to remove the nickel foam. The residue is made into a nickel foam carrier.
[0034] 2. Mix 0.5mmol of Ni(NO 3 ) 2 ·6H 2 O, 0.5mmol of Fe(NO 3 )3 9H 2 O, 0.5mmol of CO(NH 2 ) 2 and 0.45 mmol of NH 4 Add F into 40mL of deionized water and stir well to make salt solution A.
[0035] 3. Transfer the prepared salt solution A above to a microwave synthesizer connected to a reflux device. The microwave temperature is 90°C, the time is 10 minutes, and the power is 700W. After the reaction is completed, take it out and let it cool naturally, and then filter. Take the filter residue.
[0036] 4. Wash the filter residue with absolute ethanol until the filtrate is colorless. Then it was dried in an oven at 60°C for 10 h to obtain NiFe-LDH.
[0037] ...
Embodiment 2
[0043] 1. Put nickel foam (10mm×13mm×0.3mm) into 37wt% hydrochloric acid solution and ultrasonicate for 5 minutes to remove the NiO layer on the surface, then ultrasonically in deionized water and absolute ethanol for 9 minutes each to remove the nickel foam. The residue is made into a nickel foam carrier.
[0044] 2. Mix 0.5mmol of Ni(NO 3 ) 2 ·6H 2 O, 1mmol of Fe(NO 3 ) 3 9H 2 O, 0.5mmol of CO(NH 2 ) 2 and 0.45 mmol of NH 4 Add F into 40mL of deionized water and stir well to make salt solution A.
[0045] 3. Transfer the prepared salt solution A above to a microwave synthesizer connected to a reflux device. The microwave temperature is 105°C, the time is 20 minutes, and the power is 700W. After the reaction is completed, take it out and let it cool naturally, and then filter. Take the filter residue.
[0046] 4. Wash the filter residue with absolute ethanol until the filtrate is colorless. Then it was dried in an oven at 65°C for 9h to obtain NiFe-LDH.
[0047] 5...
Embodiment 3
[0053] 1. Put nickel foam (10mm×13mm×0.3mm) into 37wt% hydrochloric acid solution and ultrasonicate for 6 minutes to remove the NiO layer on the surface, then ultrasonically in deionized water and absolute ethanol for 10 minutes each to remove the nickel foam. The residue is made into a nickel foam carrier.
[0054] 2. Mix 0.5mmol of Ni(NO 3 ) 2 ·6H 2 O, 1.5mmol of Fe(NO 3 ) 3 9H 2 O, 0.5mmol of CO(NH 2 ) 2 and 0.45 mmol of NH 4 Add F into 40mL of deionized water and stir well to make salt solution A.
[0055] 3. Transfer the above-prepared salt solution A to a microwave synthesizer connected to a reflux device. The microwave temperature is 120°C, the time is 30 minutes, and the power is 700W. After the reaction is completed, take it out and let it cool naturally, and then filter. Take the filter residue.
[0056] 4. Wash the filter residue with absolute ethanol until the filtrate is colorless, and then dry it in an oven at 70°C for 8 hours to obtain NiFe-LDH.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com