Method and kit for detecting five immunosuppressants in dried blood spot
An immunosuppressant and dried blood film technology, which is applied in the field of detection of five immunosuppressants in dried blood film, can solve the problem of increasing the physiological burden and economic burden of patients and their families, the inability to monitor the five kinds of immunosuppressant therapeutic drugs, and not including Mycophenolic acid and other problems, to achieve the effect of facilitating individualized drug regimens, saving operating time, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
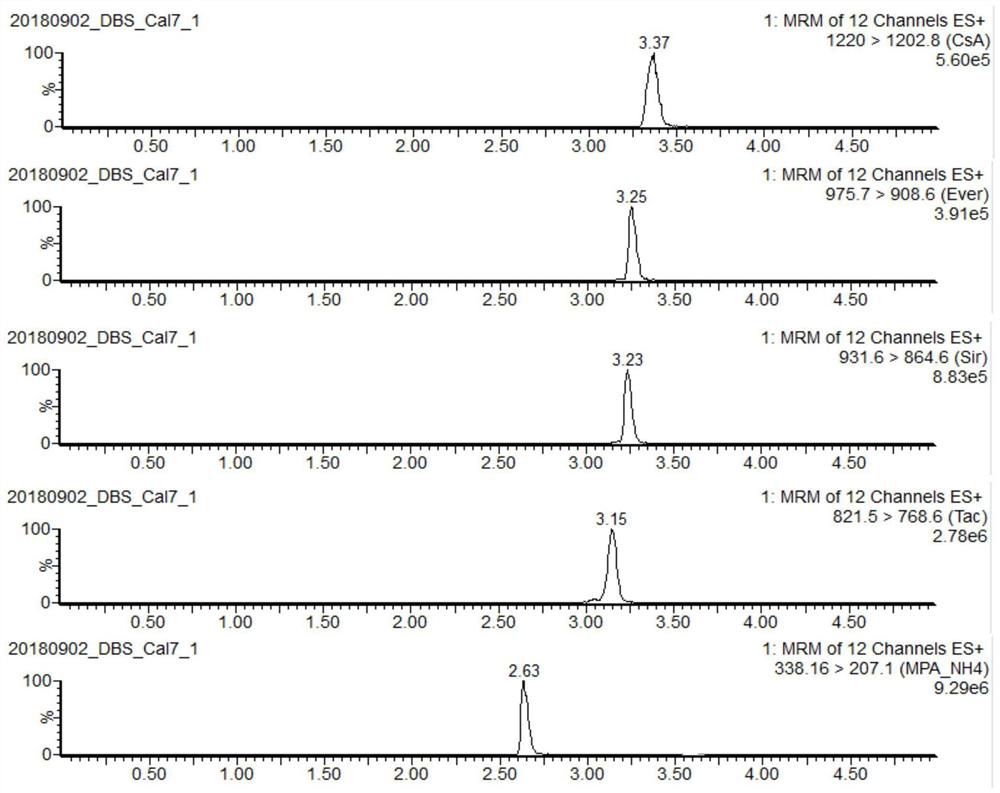
[0092] Example 1. Rapid detection kit and detection method for immunosuppressants in dried blood slices
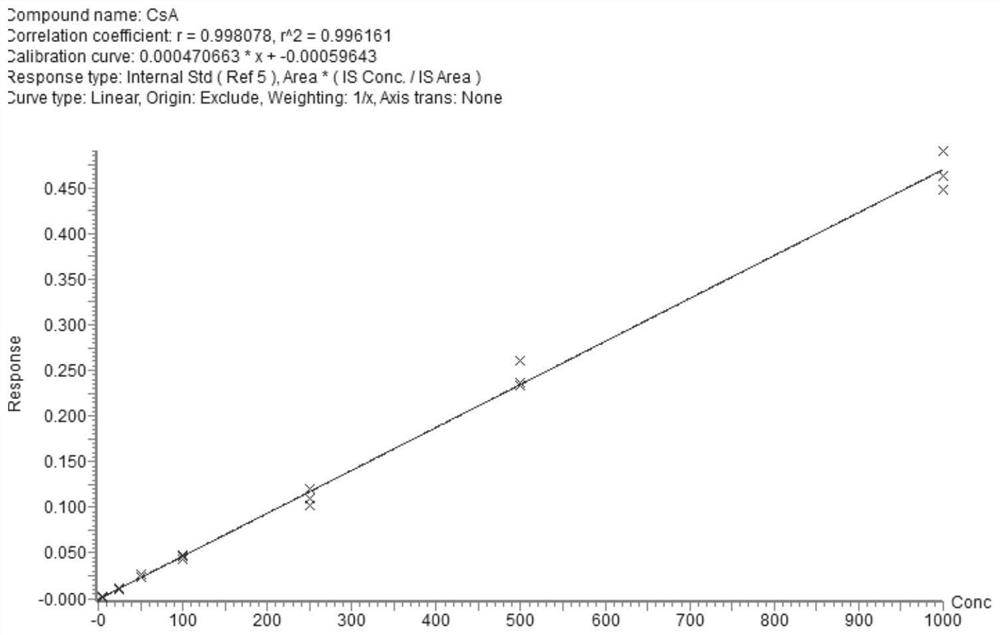
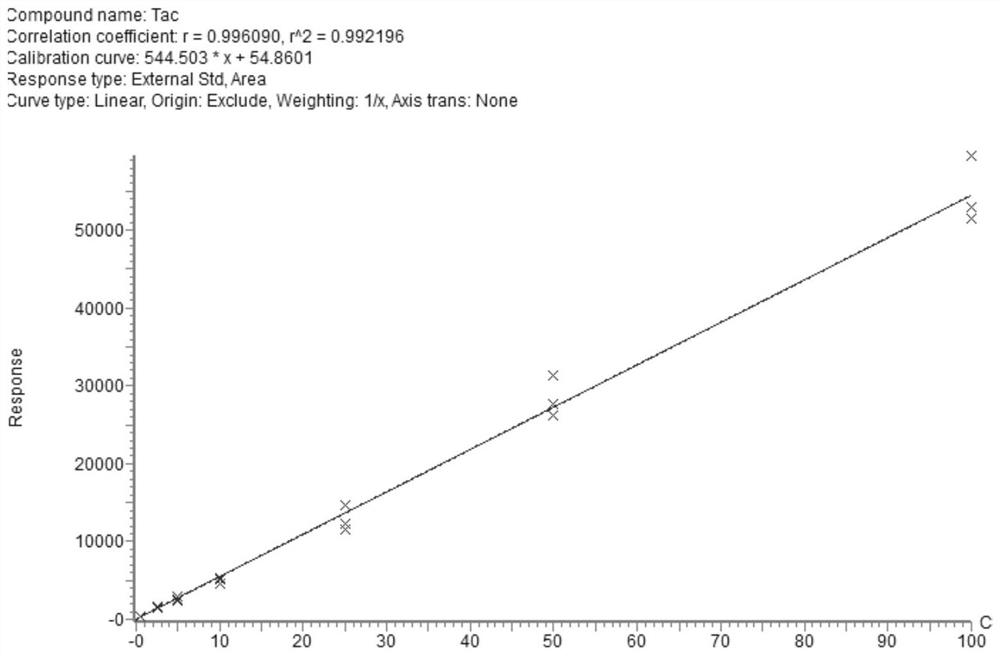
[0093] The rapid detection indicators of the kit provided in this example include: cyclosporine A, tacrolimus, sirolimus, everolimus and mycophenolic acid.
[0094] The contents of the rapid detection kit for immunosuppressants in dried blood slices provided in the present invention include:
[0095] Table 1 Kit Composition
[0096]
[0097]
[0098] Store the kit at 2-8°C in the dark.
[0099] Explanation: Since tacrolimus is relatively stable in the process of pretreatment preparation and mass spectrometry detection, it is less disturbed, and the external standard method is used for quantification, and the detection results meet the requirements. Under the condition that the detection requirements are met, the external standard method can be used to quantify tacrolimus, which can reduce the cost of sample detection. Therefore, the tacrolimus internal standard is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com