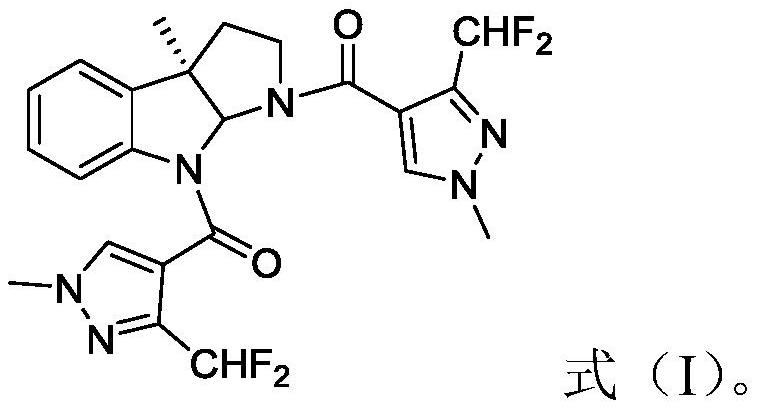
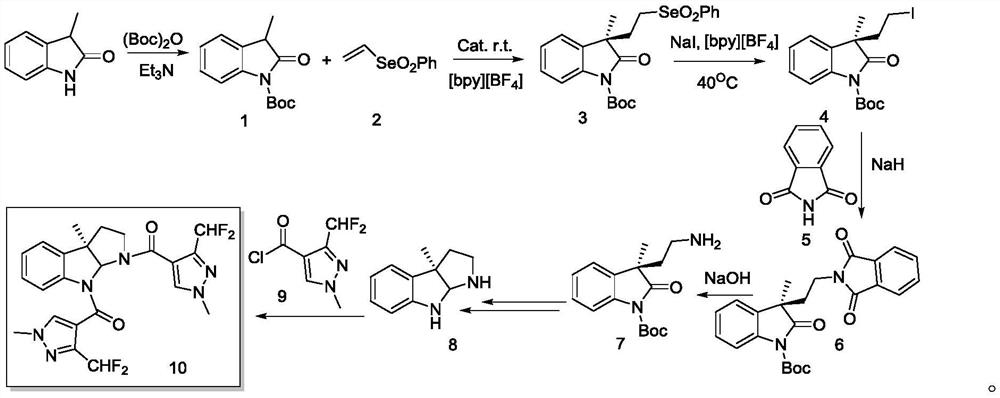
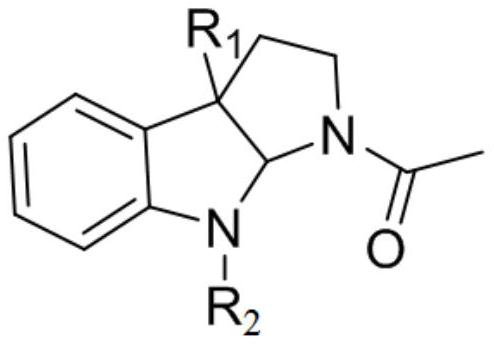
Chiral indole compound and preparation method and ship antifouling application thereof
A compound and indole technology, applied in the field of chiral indole compounds, their preparation and ship antifouling application, can solve the problems of increasing cost, polluting the environment, waste of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of intermediate compound 1
[0032]
[0033] Weigh 1.47g (10mmol) of 3-methyloxindole in a 250mL dry round bottom flask, add 30mL DCM, stir at room temperature for 15min to dissolve completely, place the reaction solution in an ice bath at 0°C, and then use Slowly add 100mL of Boc anhydride dropwise into the constant pressure funnel, and after the dropwise addition is complete, move the reaction solution to room temperature and stir for 30min. Add 50mL water to dilute, use anhydrous K 2 CO 3 or anhydrous Na 2 CO 3 The pH value of the solid is adjusted, and the pH value of the solution is detected while stirring, and the pH value of the solution is adjusted between 7 and 8. The whole system was moved to room temperature, extracted with ethyl acetate (3×100 mL), combined the organic phases, washed with saturated sodium chloride (3×50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 2.24 g of compound 1 (91% ...
Embodiment 2
[0052] Embodiment 2: Determination of bactericidal activity to synthetic compounds
[0053] The antibacterial activity of target product 10 against Staphylococcus aureus and Escherichia coli was determined by microdilution method. All strains were purchased from China General Microorganism Culture Collection Center.
[0054] Using the test tube double dilution method, take a sterilized test tube added with 2 mL of liquid medium, use the test tube inoculated without the test compound as the positive control, and use the test tube without the test compound and inoculated as the negative control. The initial mass concentration of the drug is 1g / L, which is diluted by two times successively and mixed evenly. Add 200 μL of bacterial suspension to each test tube to make the final bacterial concentration 10 4 ~10 5 cfu / mL (cfu is colony forming unit, 1cfu refers to a single colony formed on the agar plate after cultivation). After 24 hours of shaking culture, the minimum inhibito...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com