Sustained-release drug delivery system for Alzheimer's disease
A technology of senile dementia and drug delivery system, which can be applied to nervous system diseases, drug combinations, pharmaceutical formulations, etc., and can solve problems such as uneducated regulation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
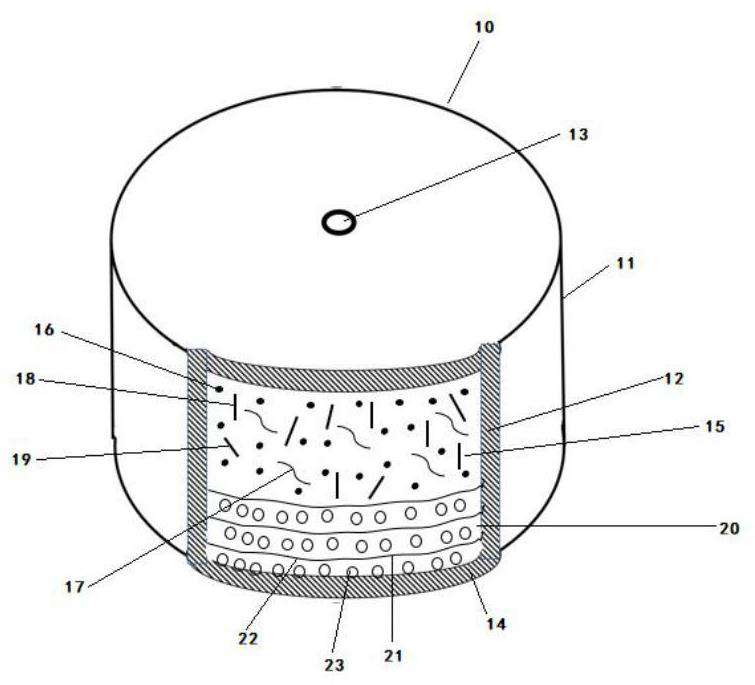
[0033] Formulation 10 was manufactured by standard manufacturing methods. For example, in one method of manufacture, a drug and other ingredients comprising a drug formulation are uniformly mixed and compressed into a solid formulation. The compressed formulation has dimensions corresponding to the internal dimensions of the area occupied by the drug formulation in formulation 10 . The formulation also corresponds to the size of the propellant it is in contact with. In this manufacturing method, the drug and other ingredients containing the ingredients are mixed with a solvent and mixed into a solid or semi-solid form by conventional methods such as ball milling, calendering, stirring or rolling, and then pressed into a predetermined shape. In the next step, the propellant composition is brought into contact with the pharmaceutical composition. The pharmaceutical composition, propellant composition can be compressed by using a conventional bilayer tablet press. The pharmace...
Embodiment 1
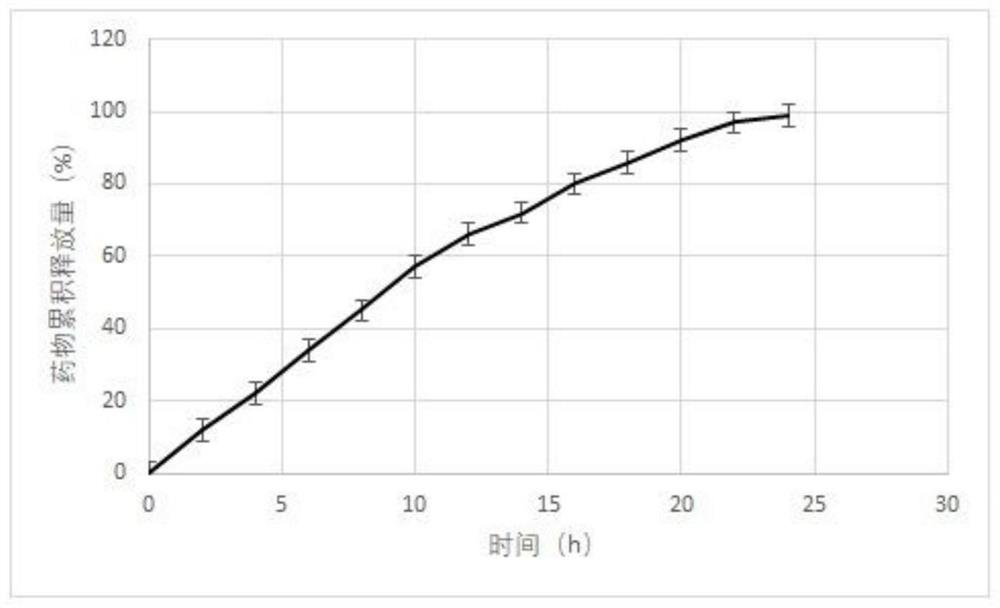
[0038] An osmotic formulation for controlled and sustained release memantine prepared as follows: 200 g of memantine, 300 g of hydroxypropylcellulose with a molecular weight of 60,000, 295 g of carboxymethylcellulose with a molecular weight of 90,000 Sodium and 100 g of polyvinylpyrrolidone with a molecular weight of 40,000 were mixed and sieved through a 40 mesh. The sieved composition was then placed in a Glatt fluid bed granulator, mixed and heated slightly to 35°C to produce a homogeneous blended powder. Next, a binder consisting of 100 g of polyvinylpyrrolidone (40000 molecular weight in 400 ml of purified water) was sprayed onto the fluidized powder.
[0039] After the spray cycle begins, the process is monitored to ensure uniformity. The granules were dried in situ and sieved through a 16 mesh screen. Next, 5 g of magnesium stearate was sieved through an 80 mesh screen, added to the granules in the blender, and blended into a homogeneous composition. The composition ...
Embodiment 2
[0044] The preparation for administering a drug to a patient according to claim 1, wherein the preparation comprises a certain amount of an oral drug having a molecular weight of 10,000 to 300,000, hydroxypropylcellulose, polyvinylpyrrolidone and carboxymethylcellulose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com