Element-doped transition metal sulfide ultrathin sheet and preparation and application thereof
A transition metal and element doping technology, applied in the direction of hydrocarbon production from carbon oxides, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention discloses a method for preparing an element-doped transition metal sulfide ultrathin sheet, which comprises the following steps:
[0041]S1. Disperse the salt, doping element and transition metal source in water or solvent for heating reaction, and obtain the precursor after drying;
[0042] S2. Using sulfur powder as a sulfur source, calcining the precursor in an inert atmosphere at 150-400° C. to obtain element-doped transition metal sulfide ultrathin flakes.
[0043] In the present invention, the mass ratio of the salt, doping element and transition metal source is 100-1000:1-500:100-1000; in some specific embodiments, the salt, doping element and transition metal The mass ratio of the source is 200-500:3-100:200-500; the mass ratio of the salt, doping element and transition metal source is preferably 100:200:400.
[0044] The above reaction is carried out in a solvent, which is well known to those skilled in the art, and the present invention is not p...
Embodiment 1
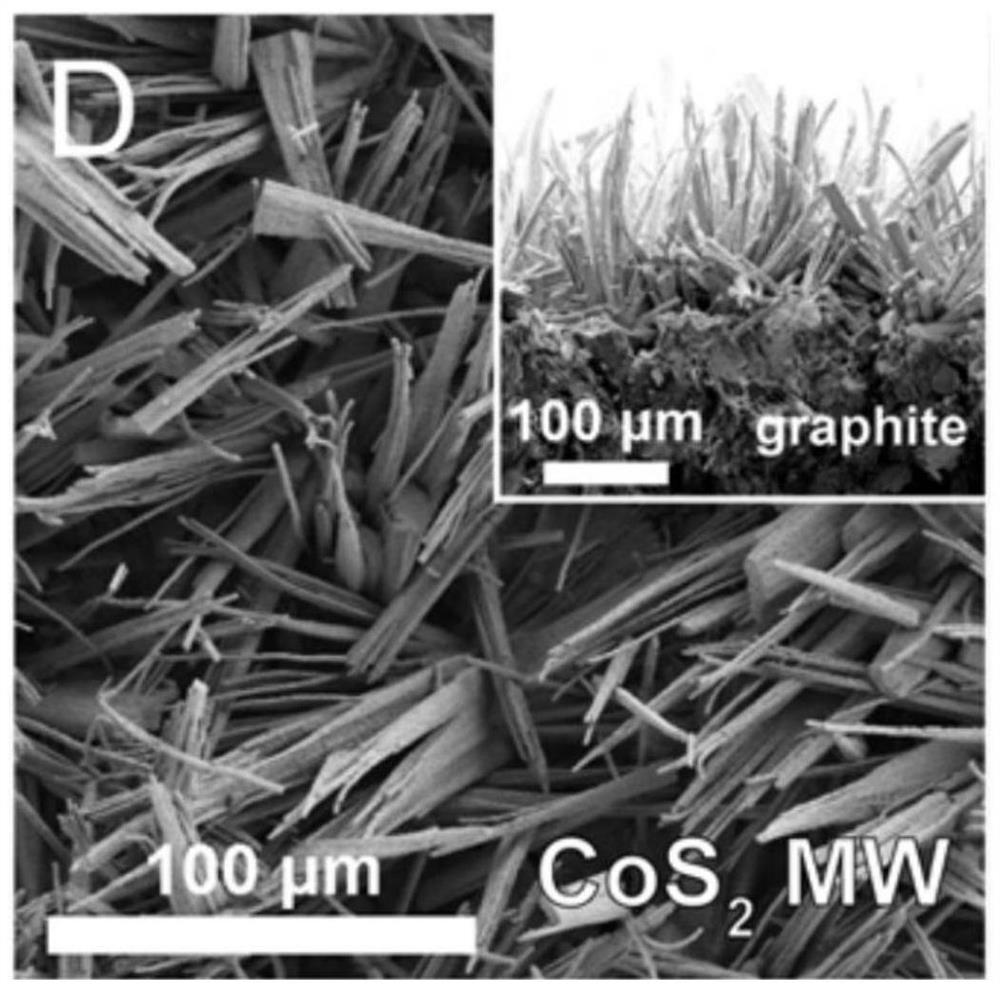
[0078] Add 100 parts of sodium sulfate, 1 part of nickel acetate tetrahydrate and 100 parts of cobalt acetate tetrahydrate to 20 parts of water, stir vigorously at room temperature, pre-cool the resulting reaction solution, put it into a freeze dryer, and freeze-dry it for 24 hours to obtain a precursor Powder: Take the obtained precursor powder in a tube furnace, put 100 parts of sulfur powder at the front end of the tube furnace, calcinate at 180-300 degrees Celsius for 1 hour in a high-purity argon atmosphere, take out the powder product after natural cooling, wash and centrifuge The sample is obtained, and the powder obtained by drying the sample in a vacuum oven at 60°C for 10 h is nickel-doped cobalt disulfide ultrathin flakes.
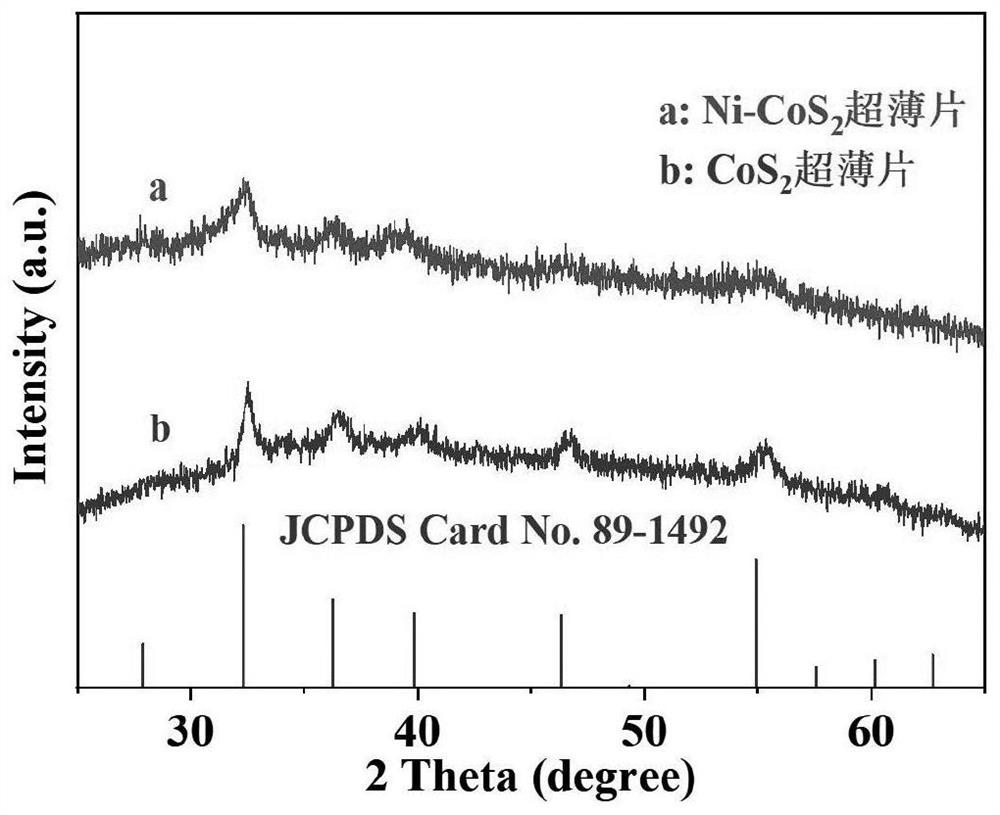
[0079] The compound prepared in Example 1 is carried out structural identification, the results are shown in Figure 3 ~ Figure 5 , image 3 The middle a curve is the X-ray diffraction (XRD diffraction) pattern of the nickel-doped cobalt disulf...
Embodiment 2
[0081] Add 1000 parts of sodium sulfate, 500 parts of nickel acetate tetrahydrate and 1000 parts of cobalt acetate tetrahydrate to 40 parts of water, stir vigorously at room temperature, pre-cool the resulting reaction solution, put it into a freeze dryer, and freeze-dry it for 24 hours to obtain a precursor Powder: Take the obtained precursor powder in a tube furnace, put 1000 parts of sulfur powder at the front end of the tube furnace, calcinate at 180-300 degrees Celsius for 1 hour in a high-purity argon atmosphere, take out the powder product after natural cooling, wash and centrifuge The sample is obtained, and the powder obtained by drying the sample in a vacuum oven at 60°C for 10 h is nickel-doped cobalt disulfide ultrathin flakes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com