Organosilicon compound and synthesis method thereof
A technology of organosilicon compounds and synthesis methods, which is applied in the fields of silicon organic compounds, organic chemistry, chemical instruments and methods, etc., and can solve problems such as unfriendly to the environment, difficulty in realizing functional groups, and uneconomical atoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
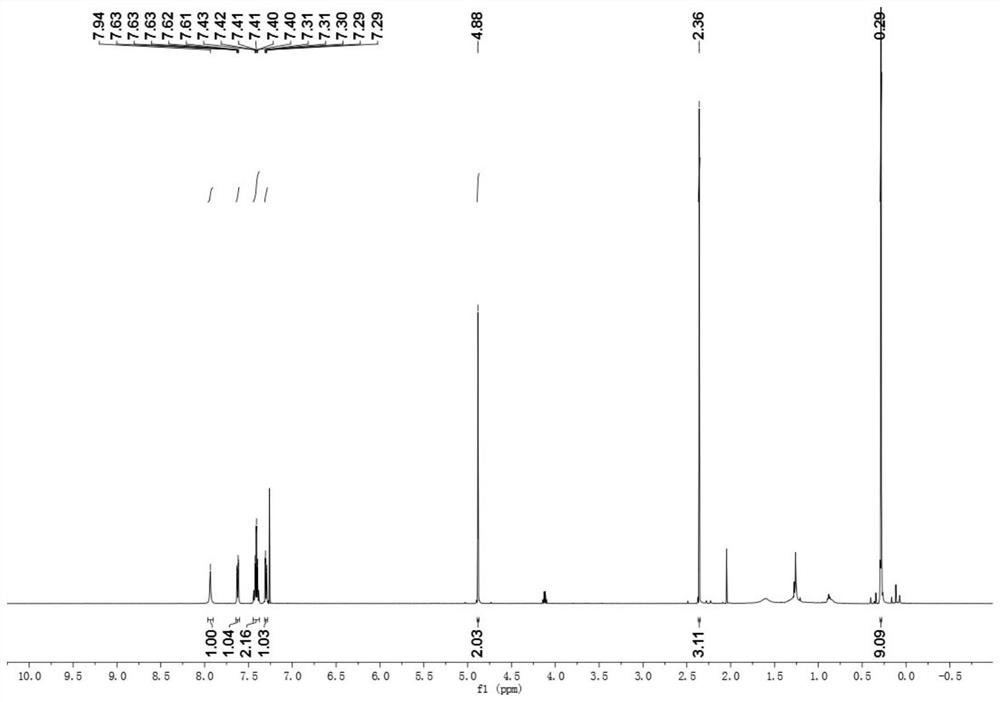
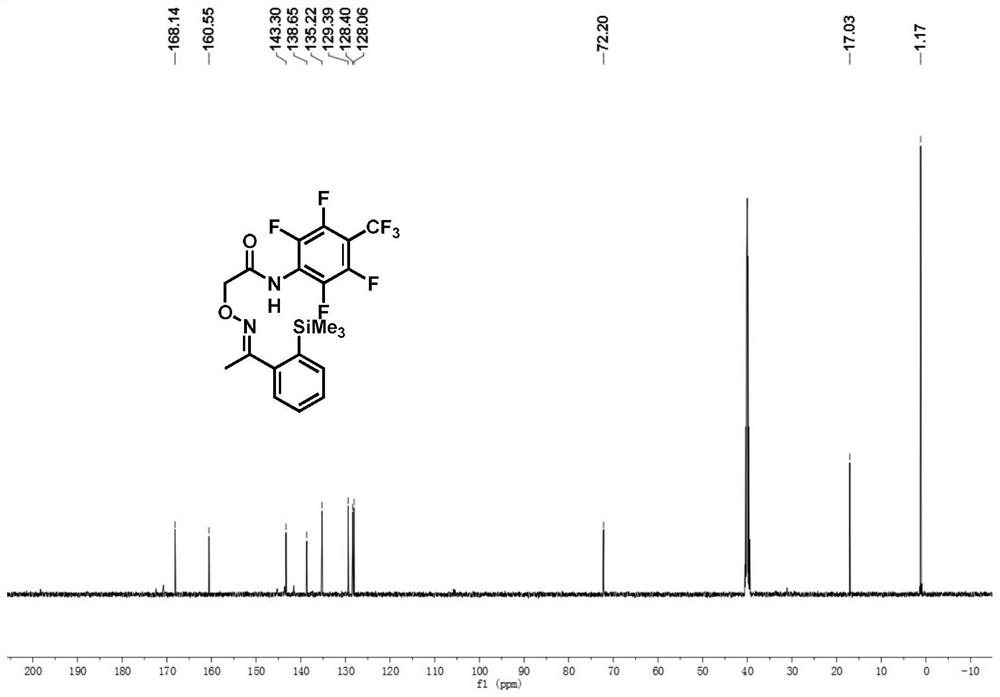
[0042] (E)-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-2-((1-(2-(trimethylsilyl)phenyl)phenylene Ethyl) amino) oxygen) acetamide synthesis:
[0043] Accurately weigh (E)-2-(((1-phenylethylidene)amino)oxy)-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl) The acetamide reactant (0.05mmol, 20.4mg) was transferred to the reaction vessel, and hexamethyldisilane (0.15mmol, 33μL), PdCl 2 (MeCN) 2(10%mmol, 1.2mg), silver fluoride (0.1mmol, 27.4mg), copper trifluoroacetate (20mmol%, 3mg), lithium fluoride (0.1mmol, 2.4mg), add dropwise 1.0mL trifluorotoluene in In a thick-walled pressure-resistant tube, tighten the stopper of the reaction tube to seal the reaction system, heat to 100°C, and react for 7 hours under the condition of stirring in an oil bath. After the reaction, the reaction solution was cooled to room temperature, filtered through short silica gel to remove insoluble impurities, washed with ethyl acetate, and the solvent was removed from the organic phase to obt...
Embodiment 2
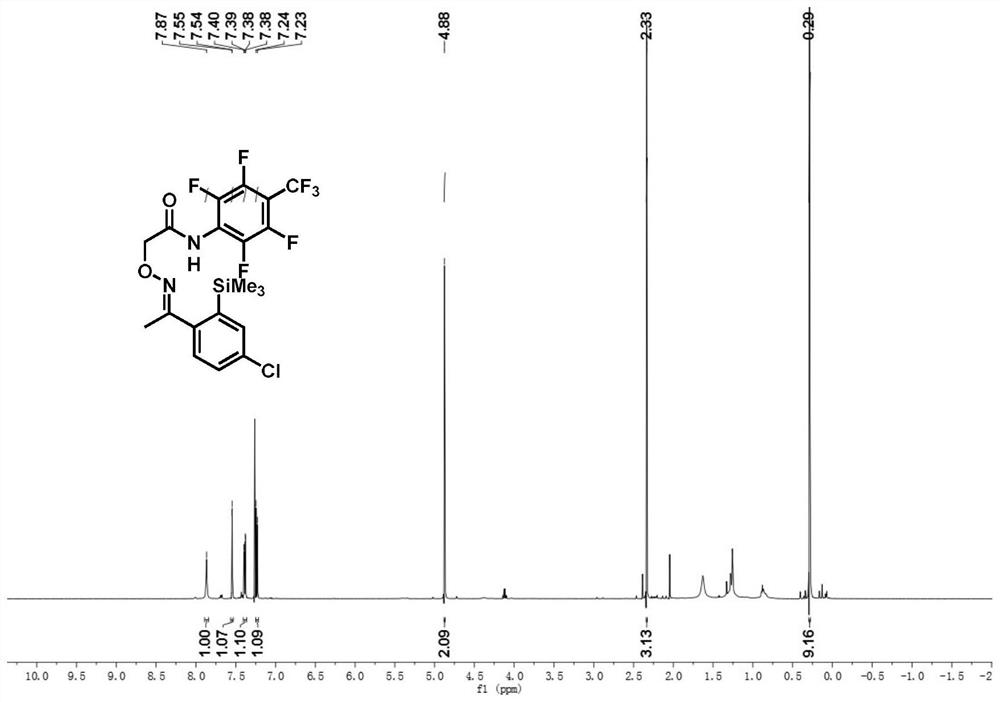
[0045] (E)-2-((1-(4-chloro-2-(trimethylsilyl)phenyl)ethylidene)amino)oxy)-N-(2,3,5,6-tetrafluoro -Synthesis of 4-(trifluoromethyl)phenyl)acetamide:
[0046] Accurately weigh (E)-2-((1-(4-chlorophenyl)ethylidene)amino)oxy)-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl Base) phenyl) acetamide reactant (0.05mmol, 22.1mg), transfer to reaction vessel, add hexamethyldisilane (0.15mmol, 33μL), PdCl 2 (MeCN) 2 (10%mmol, 1.2mg), silver fluoride (0.1mmol, 27.4mg), copper trifluoroacetate (20mmol%, 3mg), lithium fluoride (0.1mmol, 2.4mg), add dropwise 1.0mL trifluorotoluene in In a thick-walled pressure-resistant tube, tighten the stopper of the reaction tube to seal the reaction system, heat to 100°C, and react for 7 hours under the condition of stirring in an oil bath. After the reaction, the reaction solution was cooled to room temperature, filtered through short silica gel to remove insoluble impurities, washed with ethyl acetate, and the solvent was removed from the organic phase to...
Embodiment 3
[0048] (E)-2-((1-(3-chloro-2-(trimethylsilyl)phenyl)ethylidene)amino)oxy)-N-(2,3,5,6-tetrafluoro -Synthesis of 4-(trifluoromethyl)phenyl)acetamide:
[0049] Accurately weigh (E)-2-((1-(3-chlorophenyl)ethylidene)amino)oxy)-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl Base) phenyl) acetamide reactant (0.05mmol, 22.1mg), transfer to reaction vessel, add hexamethyldisilane (0.15mmol, 33μL), PdCl 2 (MeCN) 2 (10%mmol, 1.2mg), silver fluoride (0.1mmol, 27.4mg), copper trifluoroacetate (20mmol%, 3mg), lithium fluoride (0.1mmol, 2.4mg), add dropwise 1.0mL trifluorotoluene in In a thick-walled pressure-resistant tube, tighten the stopper of the reaction tube to seal the reaction system, heat to 100°C, and react for 7 hours under the condition of stirring in an oil bath. After the reaction, the reaction solution was cooled to room temperature, filtered through short silica gel to remove insoluble impurities, washed with ethyl acetate, and the solvent was removed from the organic phase to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com