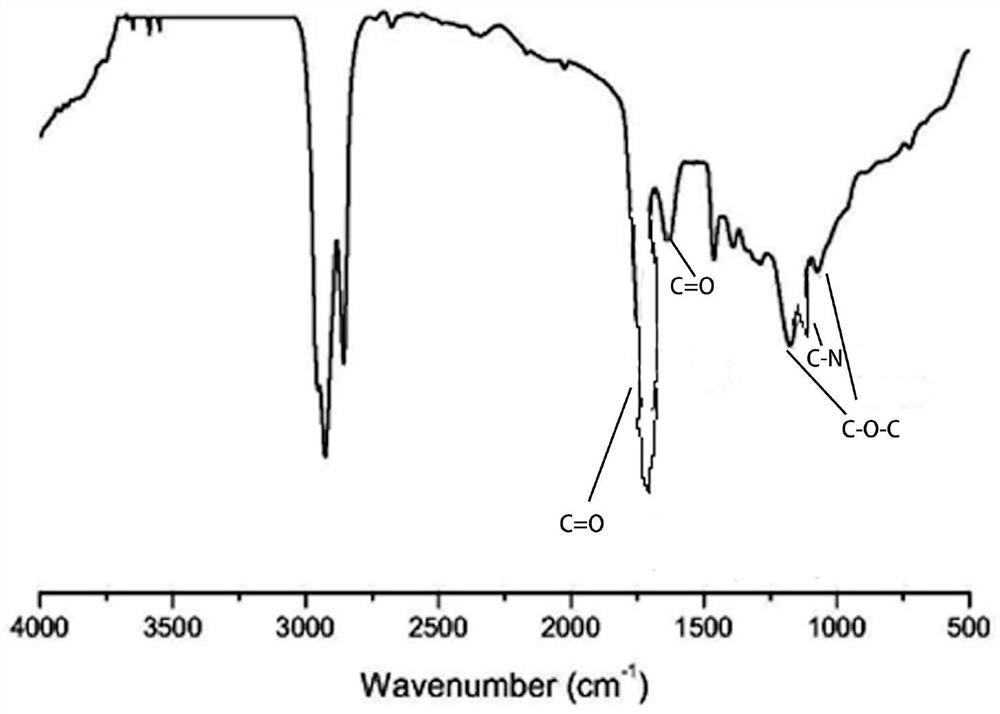
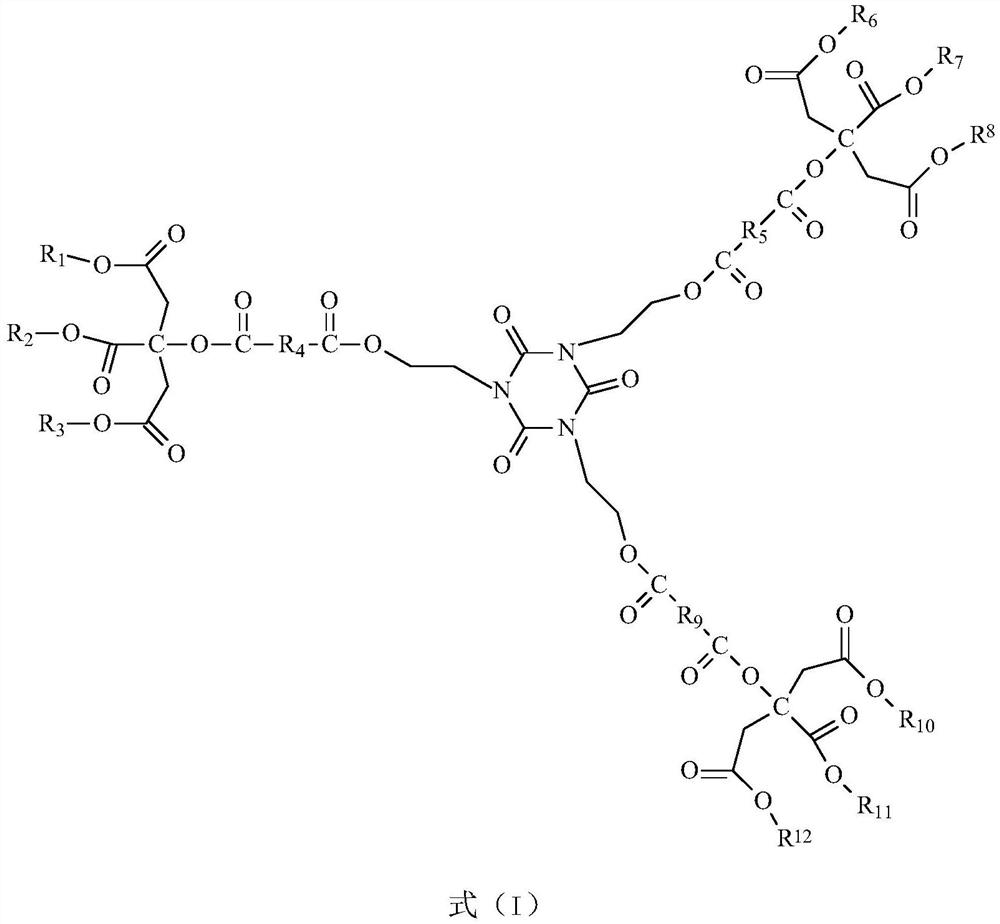
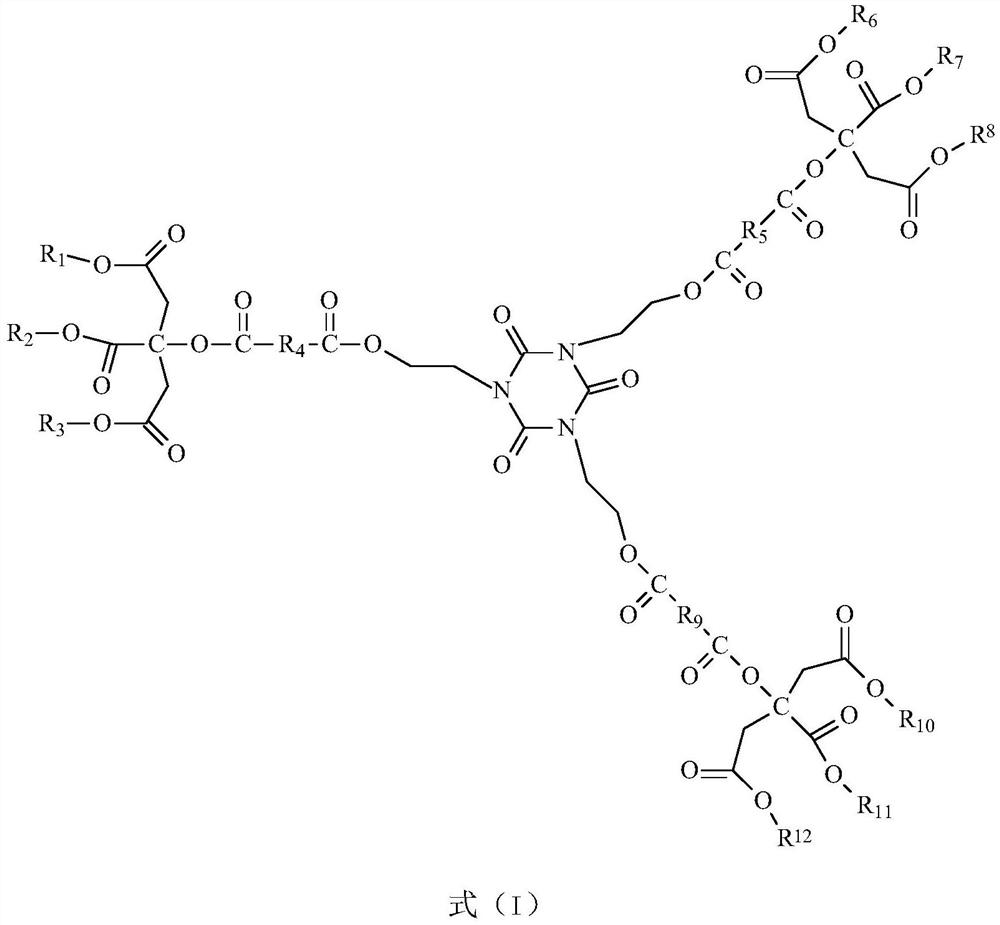
Citrate plasticizer
A technology of citrate ester and plasticizer, which is applied in the field of plastic rubber plasticizer, can solve the problems of poor solvent extraction resistance and migration resistance, insufficient migration resistance and flame retardancy, and improper number ratio. Achieve good migration resistance, meet the requirements of industrial production, and achieve the effect of low volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of tributyl citrate containing isocyanurate comprises the following steps:
[0029] 1) Preparation of tributyl citrate: add 100mol citric acid, 380mol butanols, p-benzenemethanesulfonic acid (mass fraction is 2% of the total mass of citric acid and alcohol) and 40L water-carrying agent cyclohexane in the reactor , heated to 120°C under stirring, refluxed for 4 hours, recovered excess alcohol by distillation under reduced pressure to obtain crude tributyl citrate, then washed the crude tributyl citrate with 2% sodium bicarbonate solution, removed the catalyst, and recovered the organic phase to obtain Tributyl Citrate.
[0030] 2) Preparation of carboxyl-containing tributyl citrate: add 100 mol carbon tetrachloride, 70 mol tributyl citrate and 70 mol succinic anhydride into the reaction kettle, feed nitrogen into the reaction kettle, stir and heat up to 70°C, react for 4 hours, After cooling, it was extracted and separated with ethyl acetate, and the org...
Embodiment 2
[0034] The preparation of tripentyl citrate containing isocyanurate comprises the following steps:
[0035]1) Preparation of tripentyl citrate: add 100mol citric acid, 400mol amyl alcohol, p-benzenemethanesulfonic acid (mass fraction is 2% of the total mass of citric acid and alcohol) and 40L water-carrying agent cyclohexane in the reactor , heated to 140°C under stirring, refluxed for 4 hours, and recovered excess alcohol by distillation under reduced pressure to obtain the crude product of tripentyl citrate, then washed the crude product of triamyl citrate with 2% sodium bicarbonate solution, removed the catalyst, and recovered the organic phase to obtain Tripentyl Citrate.
[0036] 2) Preparation of carboxyl-containing tripentyl citrate: add 100 mol carbon tetrachloride, 70 mol tripentyl citrate and 70 mol succinic anhydride into the reaction kettle, feed nitrogen into the reaction kettle, stir and heat up to 70° C., react for 4 hours, After cooling, it was extracted and s...
Embodiment 3
[0040] The preparation of trioctyl citrate containing isocyanurate comprises the following steps:
[0041] 1) Preparation of trioctyl citrate: add 100mol citric acid, 380mol octanol, p-benzenemethanesulfonic acid (mass fraction is 2% of the total mass of citric acid and alcohol) and 40L water-carrying agent cyclohexane in the reactor , heated to 150°C under stirring, refluxed for 4 hours, recovered excess alcohol by distillation under reduced pressure to obtain crude trioctyl citrate, then washed the crude trioctyl citrate with 2% sodium bicarbonate solution, removed the catalyst, and recovered the organic phase to obtain Trioctyl Citrate.
[0042] 2) Preparation of carboxyl-containing trioctyl citrate: add 100 mol carbon tetrachloride, 70 mol trioctyl citrate and 70 mol adipic anhydride into the reaction kettle, feed nitrogen into the reaction kettle, stir and heat up to 75 ° C, react for 5 hours, After cooling, it was extracted and separated with ethyl acetate, and the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com