Cross-genus virulent bacteriophage, preparation process and application of cross-genus virulent bacteriophage in prevention and treatment of swine paratyphoid fever and pullorum disease
A porcine paratyphoid, preparation technology, applied in the field of microbiology, can solve the problems of limited application range, no therapeutic effect, weak cracking effect, etc., achieve the effects of low production cost, broaden the scope of sterilization, and restore appetite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
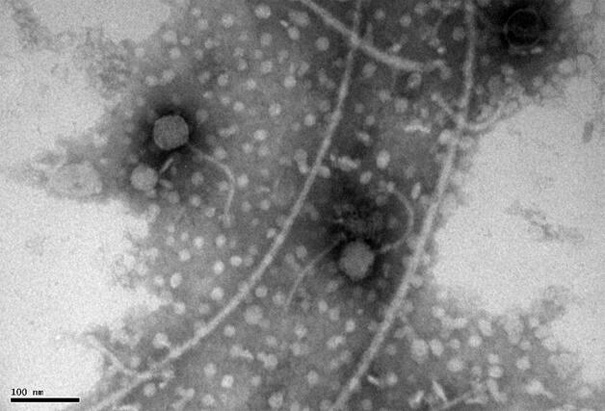
[0044] Example 1 Isolation of a virulent phage XPAR_CPS01 that cross-genus infects Salmonella pullorum and Salmonella choleraesuis
[0045] Sample treatment: Collect feces from pigs infected with paratyphoid fever from a pig farm in Yantai City, Shandong Province, soak in sterile water overnight in a 37 °C incubator, filter out excess impurities with gauze, filter and sterilize at 0.22 μM, and then use it for processing sample.
[0046] Preparation of host bacteria: Pick a single colony of Salmonella choleraesuis virulent strain C78-2 and inoculate it in 100 mL of LB broth, culture with shaking at 180 r / min at 37°C for 16-18 h to obtain a suspension of the host bacteria, and set aside.
[0047] Bacteriophage proliferation: Take 10 mL of the above-mentioned treated sample and add it to the above-mentioned Erlenmeyer flask containing the host bacterial solution. After mixing, shake and cultivate overnight in a 37°C air bath shaker at 160rpm to obtain a phage proliferation soluti...
Embodiment 2
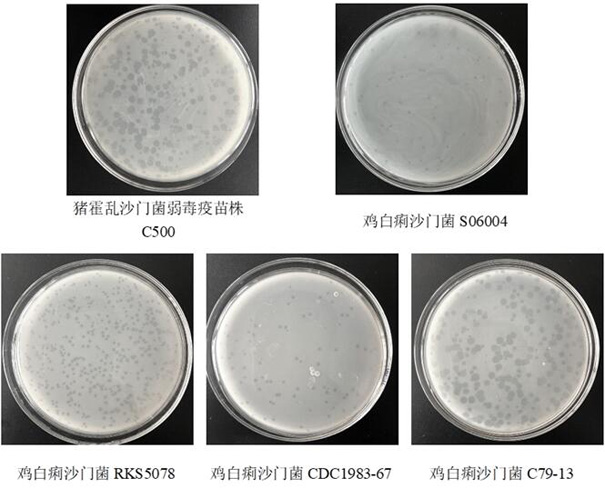
[0053] Example 2 Determination of cleavage profile of bacteriophage XPAR_CPS01
[0054] 5 mL of LB semi-solid medium was mixed with the host bacteria in the logarithmic phase of Salmonella choleraesuis virulent strain C78-2, attenuated vaccine strain C500, Salmonella pullorum C79-13, S06004, RKS5078, and CDC1983-67 (100 μl, OD =1.0) and mix well, pour it into a solid LB plate to form a double-layer plate, after standing still, take 200 μL of the above-mentioned purified phage and spread it on the plate, after it dries, culture it upside down at 37°C overnight, and observe whether there are lysis spots . The results showed that the Salmonella choleraesuis attenuated vaccine strain C500 (with a plaque diameter of 0.43 mm), Salmonella pullorum C79-13 (with a plaque diameter of 0.50 mm), S06004 (with a plaque diameter of 0.05 mm), The plaques on the plates of RKS5078 (0.16 mm in diameter of plaque) and CDC1983-67 (0.12 mm in diameter of plaque) were extremely bright, indicating t...
Embodiment 3
[0056] Example 3 Determination of the titer of bacteriophage XPAR_CPS01
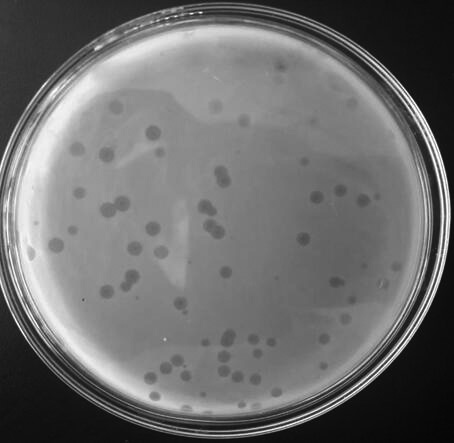
[0057] Using the Salmonella choleraesuis virus vaccine strain C500 as the host bacterium, 1 mL of the preserved phage XPAR_CPS01 stock solution was diluted 2 to 13 times by a 10-fold gradient to make dilutions of different gradients for use.
[0058] Pour 10 mL of sterilized NA medium into an empty Petri dish, and make NA agar plates after solidification. Add 0.1mL of diluents of different gradients and 1mL of host bacteria to a 15mL vial, mix with 5mL of NA medium dissolved and cooled to 60°C, and immediately pour it into the pre-prepared NA plate. As a blank control, set up three parallels. After cooling and solidifying, place it upside down in a constant temperature incubator at 37°C for 16-24 hours, read the phage titer, and determine the number of phages. Phage titer (pfu / mL) = number of plaques × dilution factor × 10. The titer of phage XPAR_CPS01 stock solution was 1.05×10 10 pfu / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com