Application of baishouwu benzophenone in preparation of uric acid reducing medicine
A technology of benzophenone and Baishouwu, which is applied in the field of medicine, can solve problems such as abnormal liver function, accelerated or irregular heartbeat, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
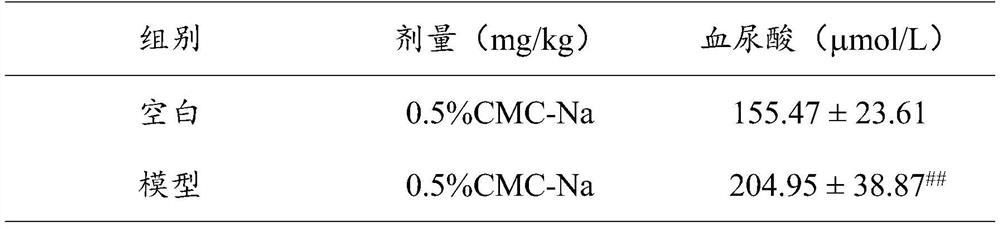
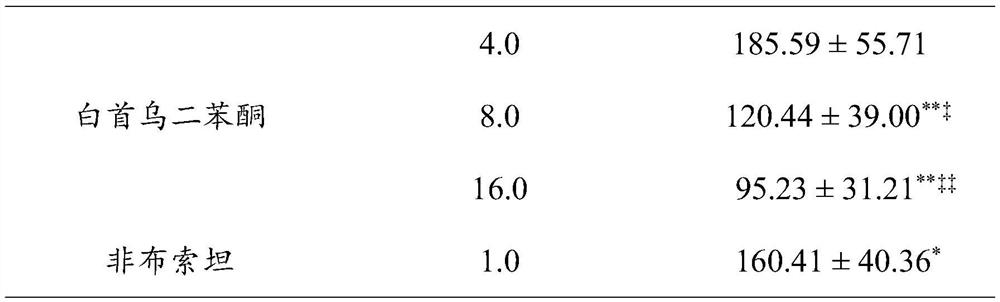
[0018] Effects of Radix Polygoni Multiflori Benzophenone on Blood Uric Acid Levels in Hyperuricemia Mice Induced by Potassium Oxonate
[0019] Test animals: male KM mice (purchased from Beijing Huafukang Biotechnology Co., Ltd., animal license number: SCXK (Beijing) 2019-0008), 18-22g.
[0020] Operation steps: the mice were divided into random groups, 10 in each group, respectively normal group, model group, each administration group and positive control group (the positive control drug was febuxostat 1.0 mg / kg, each administration group was divided into 10 mL / kg volume intragastric administration of the test drug benzophenone of Radix Polygoni Multiflori 5 times, twice a day with an interval of 8h, wherein the doses of benzophenone of Radix Polygonum multiflorum were 4mg / kg, 8mg / kg and 16mg / kg respectively; positive The treatment of the control group is the same as that of the administration group, except that the drug given is a positive control drug. 1h after the last adm...
Embodiment 2
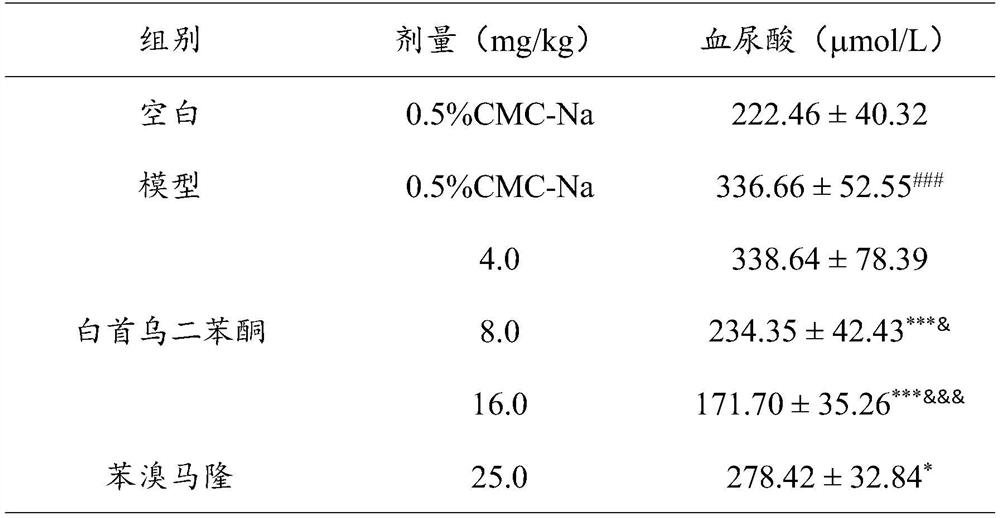
[0028] Effect of benzophenone of Radix Polygoni Multiflori on blood uric acid level in uric acid-induced hyperuricemia mice
[0029] Test animals: male KM mice (purchased from Beijing Huafukang Biotechnology Co., Ltd., animal license number: SCXK (Beijing) 2019-0008), 18-22g.
[0030] Operation steps: the mice were randomly divided into groups, 10 in each group, which were respectively the normal group, the model group, each administration group and the positive control group (the positive control drug was 25.0mg / kg benzbromarone), and each administration group was divided into 10mL / kg volume intragastric administration of the test drug benzophenone of Radix Polygoni Multiflori 5 times, twice a day with an interval of 8h, wherein the doses of benzophenone of Radix Polygonum multiflorum were 4mg / kg, 8mg / kg and 16mg / kg respectively; The treatment of the positive control group was the same as that of the treatment group, except that the drug given was benzbromarone. 0.5 hours af...
Embodiment 3
[0037] Preliminary Toxicity and Safety Evaluation of Radix Polygonum Benzophenone
[0038] Select 40 healthy ICR mice, half male and half female, weighing 18-22 g, fasting for about 12 hours before administration, and formulating a suspension with 0.5% CMC-Na of Radix Polygoni Multiflori, according to the maximum concentration, The maximum volume of 30mL / kg body weight was administered by intragastric administration, and the intragastric administration was administered once at 9:00 a.m., which was 3.0g / kg / d. After administration, continuous observation was carried out for 14 days, and the animal poisoning and death conditions were recorded. The test results are shown in Table 3 and Table 4.
[0039] Table 3 Effects of Radix Polygoni Multiflori Benzophenone Orally Administered on Body Weight of Mice on Day 0, 7, and 14 (n=10, x±s)
[0040]
[0041]
[0042] Table 4 Coefficients of organ organs (heart, kidney, spleen, liver) of mice (n=10, x±s) 14 days after intragastric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com