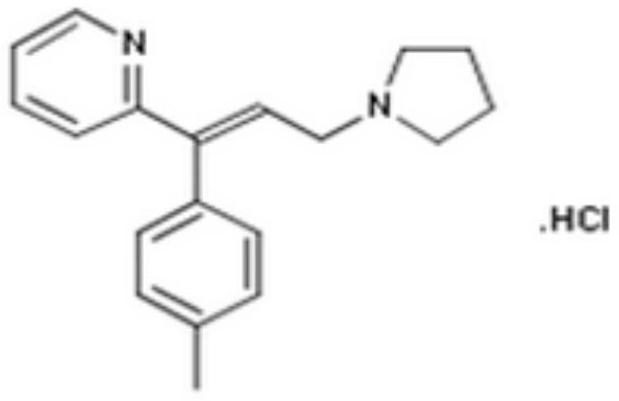
Triprolidine hydrochloride-containing oral solution and preparation method thereof
A technology of triprolidine hydrochloride and oral solution, applied in the field of pharmaceutical preparations, can solve the problems of inconvenience, short plasma half-life, reduced phenylephrine persistence, etc., and achieves a sweet taste with suitable sweet taste, controllable quality and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Raw materials Dose (g) Triprolidine Hydrochloride 0.938 Dextromethorphan Hydrobromide 9.25 Phenylephrine Hydrochloride 4.56 glycerin 125 Propylene Glycol 235 Sorbitol 100 citric acid 1.9 Sodium citrate 1.0 stevia 0.2 Potassium sorbate 1.0 orange flavor 1.0 Maltitol liquid 0.6 purified water up to 1000ml
[0025] Preparation Process:
[0026] 1. Take purified water with 65% of the total volume of the oral liquid in the liquid preparation barrel, add the prescribed amount of sodium citrate and stir, then add the prescribed amount of triprolidine hydrochloride, dextromethorphan hydrobromide, and phenylephrine hydrochloride and stir to dissolve; After adding citric acid to dissolve completely, add stevioside and sorbitol in sequence and stir to dissolve, then add glycerin, propylene glycol, essence in turn, stir until the solution is clear, and finally add potassium sorbate and malt...
Embodiment 2
[0033] Raw materials Dose (g) Triprolidine Hydrochloride 0.5 Dextromethorphan Hydrobromide 4 Phenylephrine Hydrochloride 2 glycerin 125 Propylene Glycol 235 Sorbitol 100 citric acid 2.0 Sodium citrate 1.0 stevia 0.2 Potassium sorbate 1.0 strawberry flavor 1.0 Maltitol liquid 0.6 purified water up to 1000ml
[0034] 1. Take purified water with 65% of the total volume of the oral liquid in the liquid preparation barrel, add the prescribed amount of sodium citrate and stir, then add the prescribed amount of triprolidine hydrochloride, dextromethorphan hydrobromide, and phenylephrine hydrochloride and stir to dissolve; After adding citric acid to dissolve completely, add stevioside and sorbitol in sequence and stir to dissolve, then add glycerin, propylene glycol, essence in turn, stir until the solution is clear, and finally add potassium sorbate and maltitol solution and stir to dissolve;...
Embodiment 3
[0041] Raw materials Dose (g) Triprolidine Hydrochloride 0.938 Dextromethorphan Hydrobromide 9.25 Phenylephrine Hydrochloride 4.56 glycerin 250 Propylene Glycol 235 Sorbitol 100 citric acid 1.9 Sodium citrate 1.0 Sucralose 0.2 Methylparaben 0.1 Propylparaben 0.1 strawberry flavor 1.0 Maltitol liquid 0.6 purified water up to 1000ml
[0042]1. Take purified water with 65% of the total volume of the oral liquid in the liquid preparation barrel, add the prescribed amount of sodium citrate and stir, then add the prescribed amount of triprolidine hydrochloride, dextromethorphan hydrobromide, and phenylephrine hydrochloride and stir to dissolve; After adding citric acid to dissolve completely, add stevioside and sorbitol in sequence and stir to dissolve, then add glycerin, propylene glycol, essence in turn, stir until the solution is clear, and finally add potassium sorbate and maltitol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com