FtsZ and QseC double-target antibacterial molecule as well as preparation method and application thereof
A dual-target, molecular technology, applied in the directions of antibacterial drugs, sulfonic acid amide preparation, chemical instruments and methods, etc., can solve the problems of difficult design, synthesis and application of antibacterial molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0074]Example 1 BCA-NCS5-OH (equation 2)
[0075]
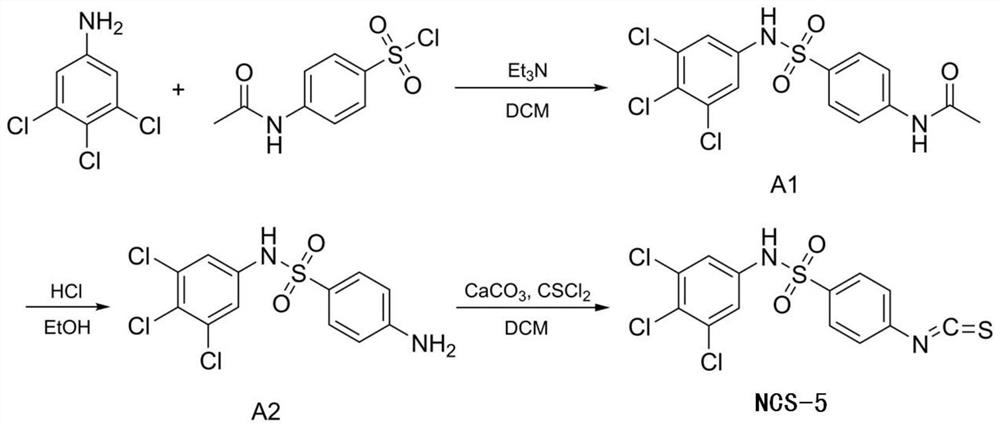
[0076]1. Synthesis of 4- (3,4,5-trichlorophenyl) benzenesulfonamide (NCS-5) (3,4,5-trichlorophenyl) benzenesulfonamide (NCS-5)figure 1 )
[0077]1) Weigh 3,4,5-trichloride 196 mg (1.0 mmol) and 233 mg (1.0 mmol) of acetaminylsylsulfonyl chloride in a 20 ml round bottom flask, and dissolved by adding 2.5 ml of dichloromethane;
[0078]2) Add 0.19 ml of triethylamine in the round bottom flask, stirring under ice water bath to 0 ° C for reaction, and then transferred to room temperature after 20 ° C for 20-30 minutes (continued to stir);
[0079]3) Room temperature for 20-30 minutes, TLC monitored reaction, 15 ml of distilled water is added to the reaction;
[0080]4) EtOAc (EtOAc) EtOAc, mixed brine, dried over an organic layer, dried over a saturated brine, filtration, filtration, and evaporative pressure reduction (-0.1MPa), the resulting solid crude product purified, The eluent is dichloromethane: methanol = 95: 5 to give brown yellow solid product...
example 2
[0102]Example 2 BCA-NCS5 (formula 3)
[0103]
[0104]1) 11.7 mg of 4-isothiocyanate-N- (3,4,5-trichlorophenyl) benzenesulfonamide (0.03 mmol) and 6.8 mg of amino α-brominnamol (0.03 mmol) 25 ml round bottom flask, and dissolved with 3 ml of dichloromethane added;
[0105]2) The reaction was stirred overnight under room temperature, filtered, evaporated. (-0.1MPa), the resulting solid crude product was purified by silica gel column: EtOAc (EtOAc) -OH (yellow oil, 5.5 mg, yield 30%);
[0106]3) Place 22 mg of BCA-NCS5-OH in a 25 ml round bottom flask, and dissolved with an additional 3.5 ml anhydrous acemitrile;
[0107]4) Add 650 mg of active manganese dioxide to the round bottom flask, stirred at room temperature for 30 minutes, thin layer chromatography;
[0108]5) After the reaction is completed, filter the core funnel, remove manganese dioxide, and the filtrate is subjected to rotation (-0.1MPa), the resulting solid crude product is purified, the eluent is n-hexane: ethyl acetate = 80: 20 The yel...
example 3
[0109]Example 3 MCA-NCS5-OH and MCA-NCS5
[0110]Molecular structure
[0111]1) MCA-NCS5-OH ((e) -4- (3- (4- (3-Hydroxy-2-MethylProp-1-EN-1-YL) Phenyl) Thioureido) -N- (3, 4, 5 -TRICHLOPHENYL) Benzenesulfonamide; Formula 4)
[0112]
[0113]2) MCA-NCS5 ((E) -4- (3- (2-methyl-3-OXOPROP-1-En-1-YL) Phenyl) Thioureido) -N- (3, 4, 5-Trichlorophenyl ) Benzenesulfonamide; formula 5)
[0114]
[0115]2. Raw materials
[0116]1) NCS-5 (4-Isothiocyanato-N- (3, 4, 5-Trichlorophenyl) Benzenesulfonamide; Formula 6), synthesisfigure 1 .
[0117]
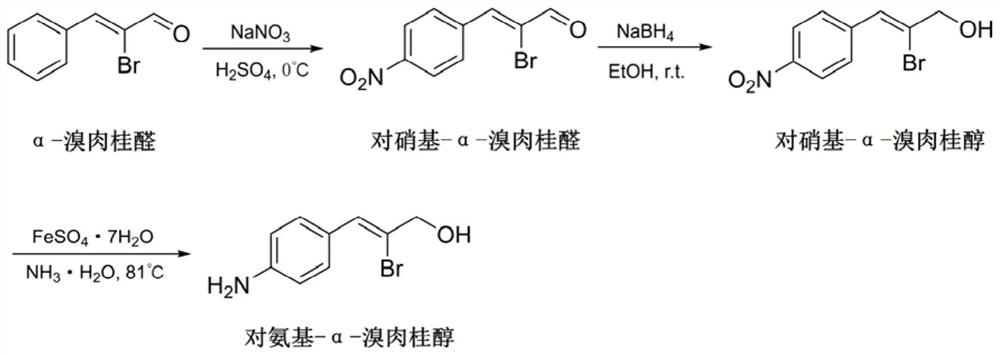
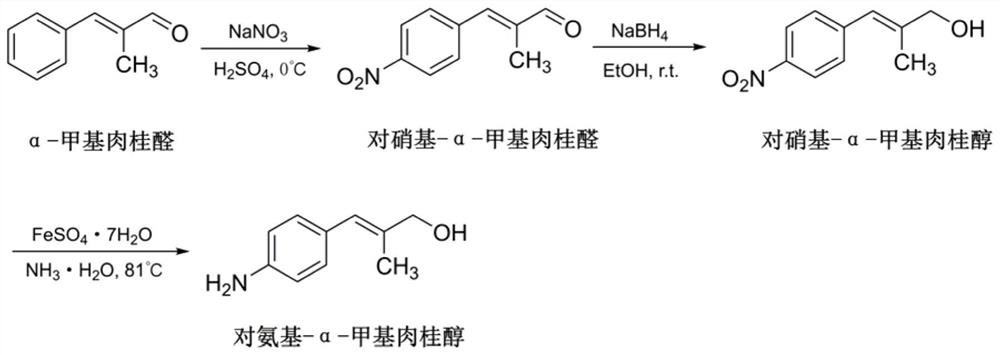
[0118]2) See the synthesis of amino alpha-methyl licking alcoholimage 3 .
[0119](3) FTSZ and Qsec dual target molecular antibacterial activity experiment
[0120]The present invention determines the minimum inhibitory concentration (MIC) of the synthesized FTSZ and Qsec dual target molecules, detecting the effect of the FTSZ and Qsec dual-target molecules on the expression level of FTSZ protein GTPase activity and bacterial virulence factor, and then evaluation Its application in ant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com