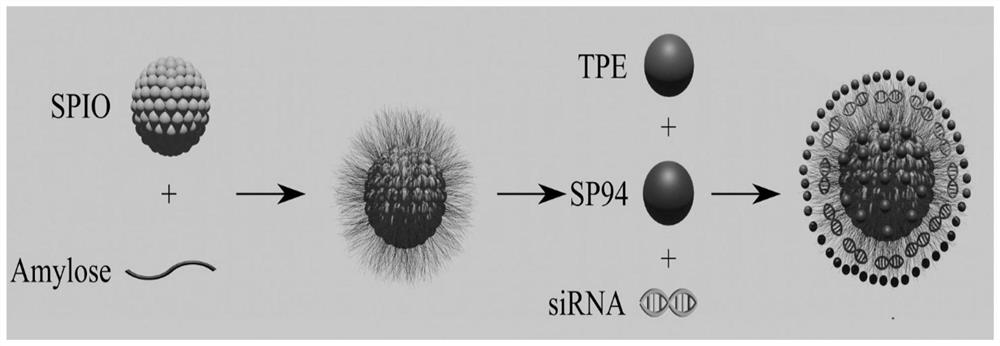
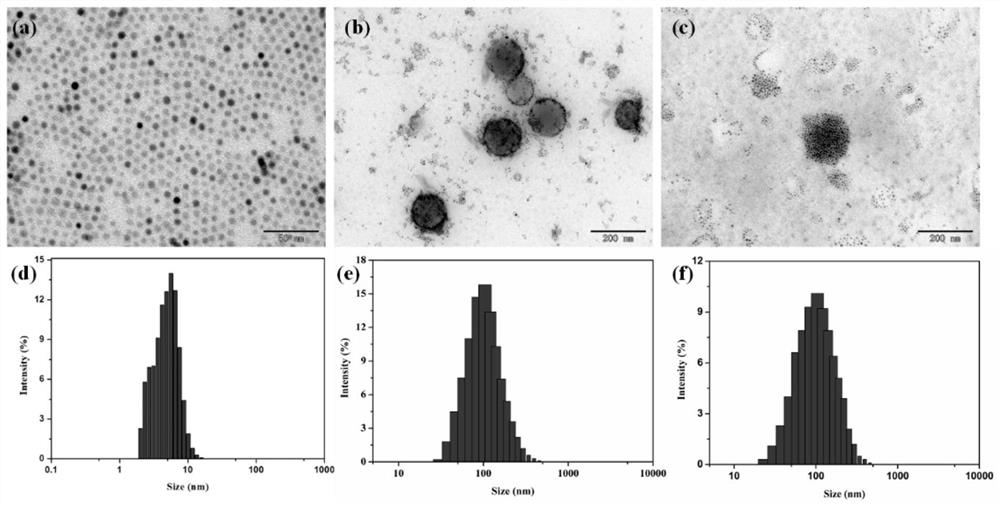
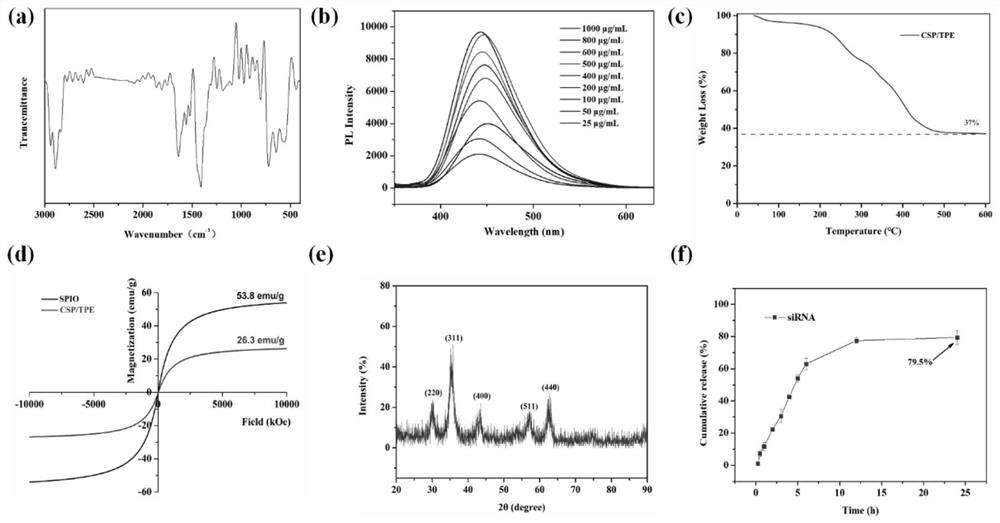
Magnetic nano-drug carrier as well as preparation method and application thereof
A magnetic nano-carrier technology, applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as refractory degradation, high toxicity, and difficulty in living body tracing, and achieve good biocompatibility and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation of quaternary ammonium cationized amylose (CA)
[0045] Weigh 0.50g of amylose and add it to 25mL of distilled water, adjust the pH to 12-14 with 4mol / L NaOH solution, heat and stir to disperse; slowly add 5mL of aqueous solution containing 0.25g of active etherification agent GTA, at 50℃ Continue to stir and react for 12 hours; after the reaction, adjust the pH to neutral with hydrochloric acid solution, transfer all the solution to a cellulose dialysis bag with a molecular weight cut-off of 8000-14000 Da, dialyze with deionized water for 3 days, filter the dialysate and freeze After drying, quaternary ammonium cationized amylose was obtained, denoted as CA.
Embodiment 2
[0046] Embodiment 2, the aqueous solution of quaternary ammonium cationized amylose and superparamagnetic iron oxide nanoparticle composite Preparation of (CA-SPIO)
[0047] Weigh 0.20g quaternary ammonium cationized amylose in a 50mL flask, add 20mL distilled water and stir to dissolve; weigh 0.20g FeCl 3 ·6H 2 O and 0.10 g FeCl 2 4H 2 O was dissolved in 5 mL of distilled water and added to the above system, and stirred under nitrogen gas for 30 minutes. The flask was placed in an 80°C water bath, and 2.5 mL of 25% ammonia water was added with a syringe under vigorous stirring, and reacted for 1 hour. After the reaction was completed, the temperature was lowered to room temperature, and the entire solution was transferred to a cellulose dialysis bag with a molecular weight cut-off of 8000-14000 Da for dialysis for 2 days. After the dialysate was centrifuged to remove insoluble matter, the upper layer was stored at 4°C to obtain an aqueous solution of a complex of quat...
Embodiment 3
[0048] Embodiment 3, quaternary ammonium cationized amylose-tetraphenylethylene-superparamagnetic iron oxide nanoparticles (CA- Preparation of SPIO-TPE)
[0049] Weigh 4mg TPE and dissolve in 4mL CH 2 Cl 2 Then add 20mg aqueous solution (CA-SPIO) of quaternary ammonium cationized amylose and superparamagnetic nanoparticle complex (CA-SPIO), mix well at room temperature, then drop into 40mL pure water while ultrasonic, after ultrasonic for 1 hour, the product is transferred into into a dialysis bag with a molecular weight cut-off of 2000Da, dialyze for 3 days, change pure water twice a day, freeze-dry the dialysate, collect the solid product and store it at 4°C for later use to obtain quaternary ammonium cationized amylose-tetraphenylethylene - Superparamagnetic iron oxide nanoparticles, denoted as CA-SPIO-TPE.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com