Monoclonal antibody specifically combined with porcine rotavirus and application thereof
A monoclonal antibody and porcine rotavirus technology, applied in the direction of antibodies, antiviral agents, antiviral immunoglobulin, etc., can solve the problems of expensive instruments, high background, and time-consuming separation and identification, and achieve rapid detection and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation, purification and identification of porcine rotavirus monoclonal antibody
[0061] 1.1 Preparation of monoclonal antibody
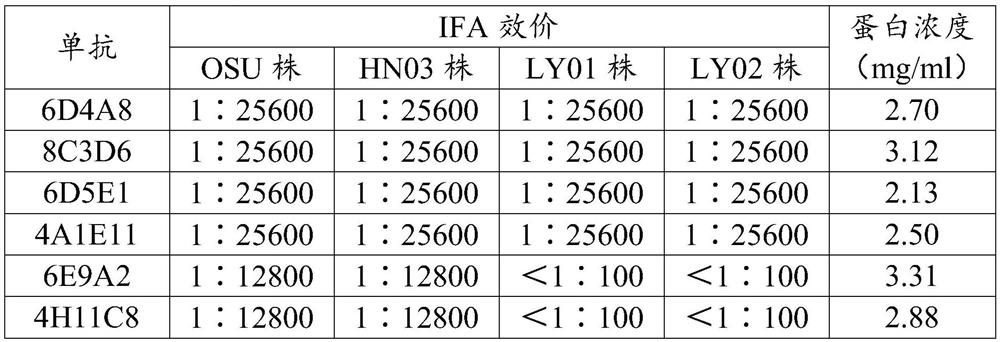
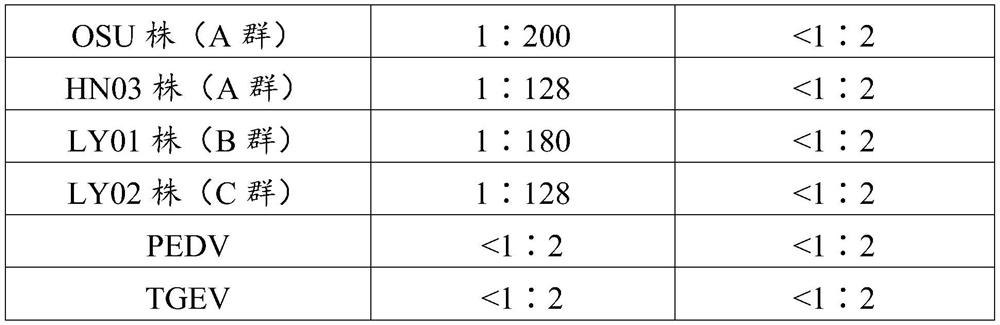
[0062] Cultivate the porcine rotavirus OSU strain (purchased from ATCC), centrifuge the harvested virus liquid at 5000 rpm for 30 minutes, get the supernatant at 32000 rpm for 2 hours, and resuspend the precipitate with PBS; prepare 55%, 65 %, 75% density sucrose solution, add it to a centrifuge tube to make a 55%-75% sucrose concentration gradient, add the precipitate suspension, centrifuge at 4°C, 32000 rpm for 2 hours, and harvest a 60%-70% concentration gradient After purification, the protein concentration was determined to be 0.98mg / ml; it was emulsified with Freund's adjuvant, and the final content was 30μg / mouse to immunize mice every 2 weeks. After immunization for 3 times, blood was collected from mice and used Indirect Immunofluorescence (IFA) (inoculate cells with PRoV until 30% of the lesions appear in the cells,...
Embodiment 2
[0104] Preparation and application of embodiment 2 kit
[0105] 2.1 Pairing studies of monoclonal antibodies
[0106] Colloidal gold detection test strips were prepared by using monoclonal antibodies 8C3D6 and 6D4A8 as gold-labeled antibodies and immobilized antibodies, respectively. Dilute the PRoV OSU strain and HN03 strain to 10 5.0 TCID 50 / ml, detection of PRoV OSU strain, HN03 strain and clinically negative feces identified by RT-PCR, the results are shown in Table 5: when using gold-labeled antibody 6D4A8 and immobilized antibody 8C3D6, the dilutions of PRoV OSU strain and HN03 strain were all positive, clinical Negative stools are weakly positive and false positive. Therefore, the gold-labeled antibody 6D4A8 and the immobilized antibody 8C3D6 were selected as the matching method for kit preparation.
[0107] Table 5 Matching results of monoclonal antibodies
[0108] immobilized antibody Gold labeled antibody OSU strain HN03 strain clinically nega...
Embodiment 3
[0154] Example 3 Effect Evaluation of Monoclonal Antibody 8C3D6 in Preventing and / or Treating Virus Infection
[0155] 3.1 Evaluation of the effect of monoclonal antibody 8C3D6 in preventing viral infection
[0156] Screen PEDV, TGEV, PRoV antigen, antibody double-negative 2-3 day old piglets 15, be divided into 3 groups at random, the monoclonal antibody 8C3D6 2mL that the first group and the second group are prepared orally in the embodiment 1 respectively, the third 2 mL of PBS buffer solution was taken orally in the first group, and 24 hours after the oral administration, the first group and the third group were all orally administered 2 mL of PRoV epidemic strain HN03 strain small intestine virus (containing 1000 minimum incidence doses), and the second group was orally administered OSU strain small intestine virus (containing 1000 Minimum incidence dose), observe the clinical symptoms of piglets every day, and observe continuously for 14 days, and evaluate the prevention...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com