High-ductility high-performance semiconductor conjugated polymer and preparation method thereof
A conjugated polymer and semiconductor technology, applied in the field of high-performance semiconductor conjugated polymer materials, high-performance semiconductor conjugated polymers and their preparation, can solve the problems of short side chains and inability to form elastic regions, and achieve improved tensile strength. The effect of extensibility, high guiding significance and good electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Synthesis of Polymer PTDPPTT-C 6 -Si 5 (m=6, p1=4, the main chain structure is DPP-thiophene)
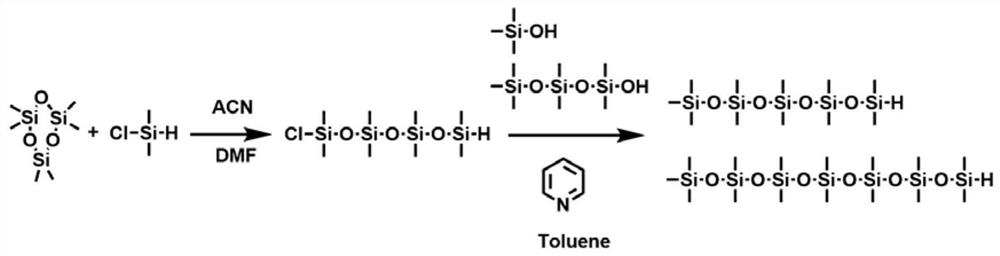
[0061] 1. If figure 1 Shown, the synthetic method of the organosiloxane group of part semiconductor conjugated polymer in the method for the present invention is: with hexamethylcyclotrisiloxane (0.25mol) and chlorodimethylsilyl hydrogen ( 0.25mol) as a raw material, with acetonitrile (40ml) as a solvent, DMF (2ml) as a catalyst, react at room temperature for 72h to obtain chlorooctamethylsilylhydrogen; Add methylsilylhydrogen (0.045mol) into dry toluene (40ml), ice-bath for 3-5min, add pyridine (5ml), dropwise within 1-2min, dissolve trimethylsilanol in toluene, gradually within 15min Add it dropwise into the reaction flask, react at room temperature for 2-3 hours, and distill under reduced pressure to obtain organosiloxane (1,1,1,3,3,5,5,7,7,9,9-undecamethylpentasilicone oxane).
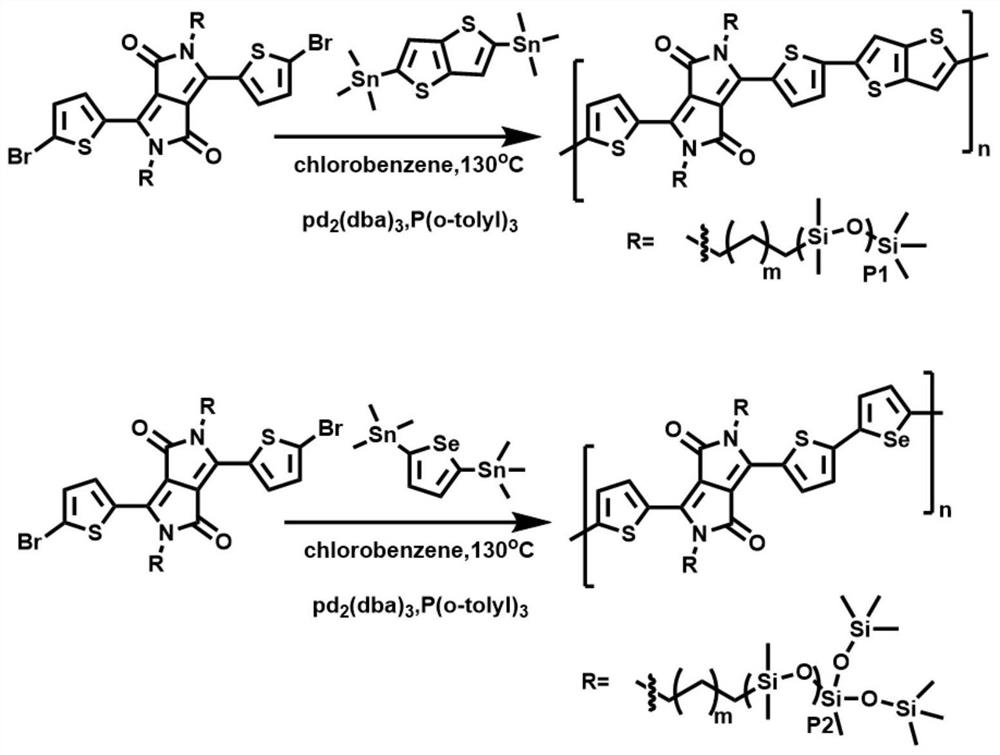
[0062] 2. Join K 2 CO 3 , DPP, and 6-bromo-1-hexene into a single-port react...
Embodiment 2
[0067] Example 2: Synthesis of polymer PTDPPTT-C 6 -Si 7 (m=6, p1=6, the main chain structure is DPP-thiophene)
[0068] 1. If figure 1 As shown, take hexamethylcyclotrisiloxane (0.25mol) and chlorodimethylsilyl hydrogen (0.25mol) as raw materials, acetonitrile (40ml) as solvent, DMF (2ml) as catalyst, react at room temperature 72h, to obtain chlorooctamethylsilylhydrogen; under the conditions of ice bath and nitrogen gas flow, add chlorooctamethylsilylhydrogen (0.045mol) into dry toluene (40ml), ice bath for 3-5min, add pyridine (5ml), the dropwise addition is completed within 1-2min, dissolve hexamethylsilanol in toluene, gradually add dropwise into the reaction bottle within 15min, react at room temperature for 2-3h, and distill under reduced pressure to obtain the final organosiloxane group (1,1,1,3,3,5,5,7,7,9,9-pentadecamethylpentasiloxane).
[0069] 2. Join K 2 CO 3 , DPP, and 6-bromo-1-hexene into a single-port reaction flask, add an appropriate amount of DMF to ...
Embodiment 3
[0074] Embodiment 3: synthetic polymer PTDPPSe-C 7 -Si 5 (m=7, p2=2, the main chain structure is monoselenophene, and the main chain and side chain structures are adjusted to obtain the optimal combination).
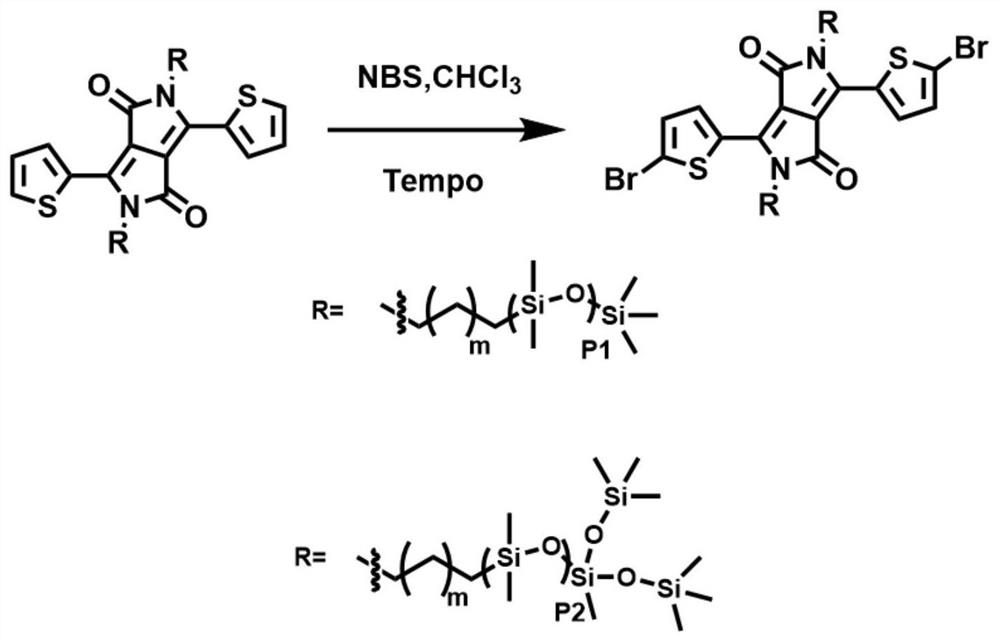
[0075] Such as figure 2 Shown, the bromination synthetic method of thiophene DPP monomer in the present embodiment is as follows:
[0076] Add 0.38mmol of unbrominated DPP monomer into a 100ml reaction bottle, add 15ml of chloroform to dissolve, add 0.1g of free radical scavenger (Tempo), then dissolve NBS (0.8mmol) in chloroform, and add to the reaction dropwise In the bottle, the dropwise addition was completed within 20 minutes, the reaction bottle was moved to room temperature, and reacted overnight; the reacted product was extracted twice with water, dried by adding anhydrous magnesium sulfate, and filtered to obtain a purple-red oily brominated monomer; The product was subjected to silica gel chromatography, using petroleum ether / ethyl acetate (100:1) as the elue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com