A praseodymium-zirconium composite oxide cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
A technology of composite oxides and cobalt-based catalysts, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical/physical processes, etc., can solve catalyst deactivation, poor stability, intolerance Sintering and other problems to achieve the effect of improving catalytic activity, promoting migration, and inhibiting the production of acetone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Weigh 7.972g of ZrO (NO 3 ) 2 ·2H 2 O, 2.921g of Co(NO 3 ) 2 ·6H 2 O, add 10ml deionized water to prepare solution #1; weigh 9.353g of citric acid in a 250ml beaker, add 10ml deionized water, stir and dissolve with a magnetic stirrer to prepare solution #2; citric acid solution # 2 Stir in a water bath at 70°C, slowly add nitrate solution #1 dropwise to the citric acid solution, and react for complexation for 0.5h; weigh 2.763g of ethylene glycol solution, slowly add it dropwise to the aforementioned mixed solution, maintain Stir in a water bath at 70 °C for 3 h to form a gel, place it in a drying oven at 105 °C for 12 h, and then calcinate at 750 °C for 4 h to obtain a CDUT-CZ catalyst. The molar composition of the catalyst is (ZrO 2 ) 0.68 (CoO1.5) 0.2 , the weight percentage composition is: zirconia is 84.9%, cobalt oxide is 15.1%.
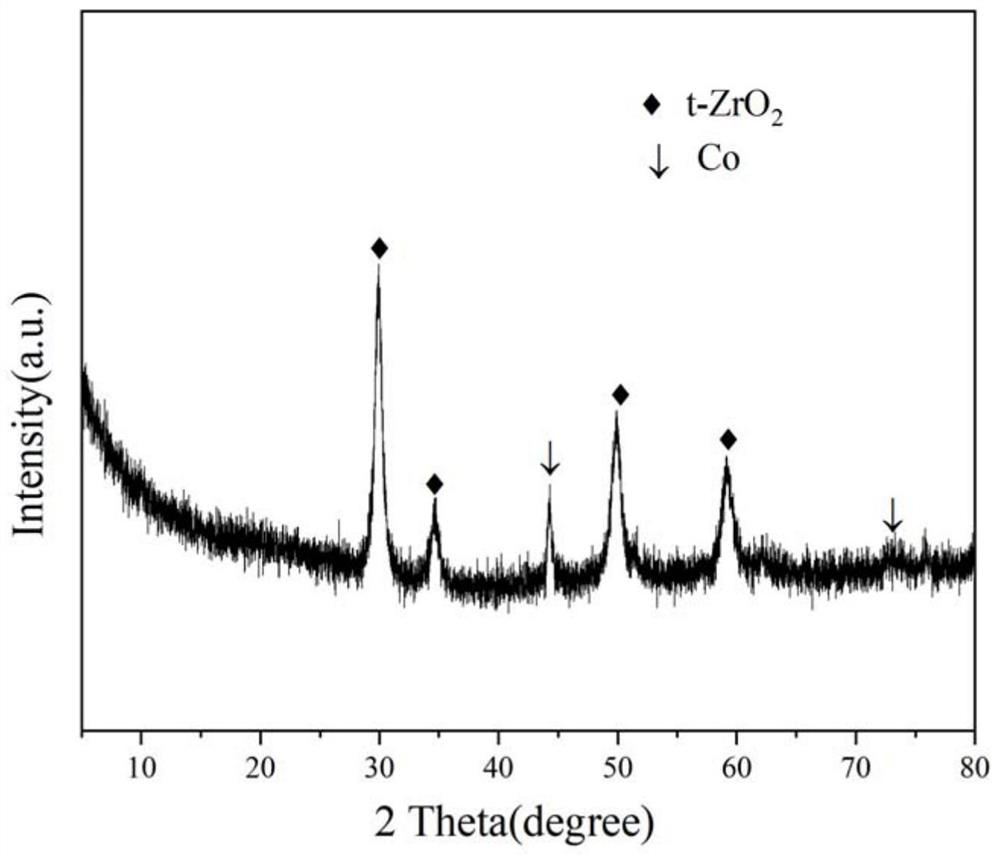
[0030] The evaluation of acetic acid autothermal reforming reaction activity was carried out in a continuous flow fixed bed re...
Embodiment 1
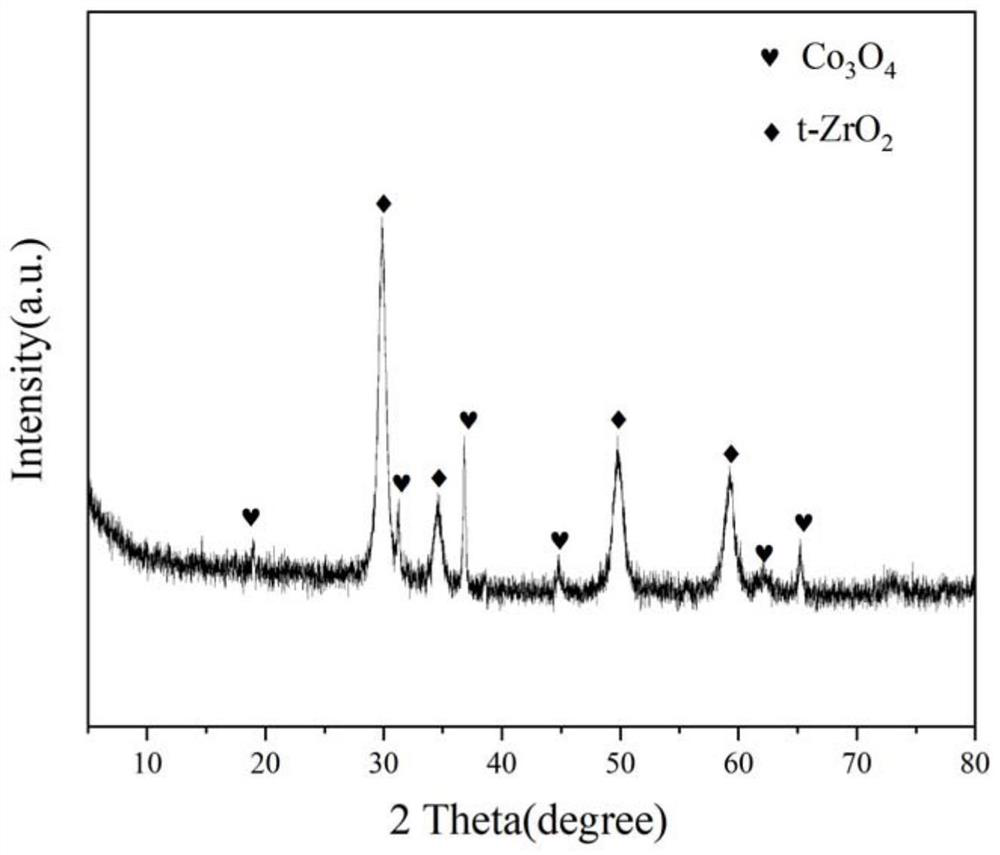
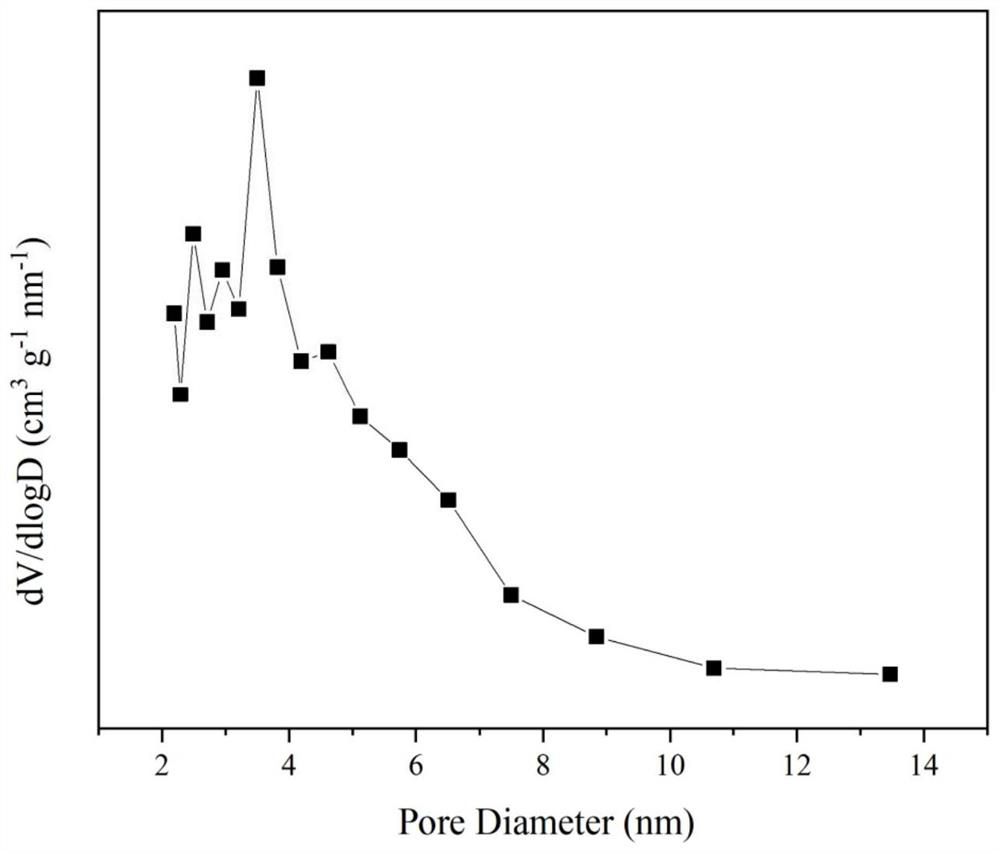
[0033] Weigh 1.451g of Pr(NO 3 ) 3 ·6H 2 O, 6.939g of ZrO (NO 3 ) 2 ·2H 2 O and 2.921g of Co(NO 3 ) 2 ·6H 2 O, add 10ml deionized water to prepare solution #1; weigh 9.117g of citric acid in a 250ml beaker, add 10ml deionized water, stir and dissolve with a magnetic stirrer, prepare solution #2; put citric acid solution # 2 Stir in a water bath at 70°C, slowly add nitrate solution #1 dropwise to the citric acid solution, and react for complexation for 0.5h; weigh 2.693g of ethylene glycol solution, slowly add it dropwise to the aforementioned mixed solution, maintain Stir in a water bath at 70°C for 3h to form a gel, place it in a drying oven at 105°C for 12h, and then calcinate at 750°C for 4h to obtain Co containing spinel phase 3 O 4 and Pr-doped tetragonal ZrO 2 The Co-Zr-Pr-O composite oxide cobalt-based catalyst, namely CDUT-CZP-I catalyst, has a typical crystal structure of the oxide as shown in the appendix. figure 1 As shown, its pore structure characterist...
Embodiment 2
[0036] Weigh 2.810g of Pr(NO 3 ) 3 ·6H 2 O, 5.976g of ZrO (NO 3 ) 2 ·2H 2 O and 2.912g of Co(NO 3 ) 2 ·6H 2O, add 10ml deionized water to prepare solution #1; weigh 8.892g of citric acid in a 250ml beaker, add 10ml deionized water, stir and dissolve with a magnetic stirrer, prepare solution #2; citric acid solution # 2 Stir in a water bath at 70°C, slowly add nitrate solution #1 dropwise to the citric acid solution, and react for 0.5h; weigh 2.627g of ethylene glycol solution, slowly add it dropwise to the aforementioned mixed solution, maintain Stir in a water bath at 70°C for 3h to form a gel, place it in a drying oven at 105°C for 12h, and then calcinate at 750°C for 4h to obtain a Co containing spinel phase 3 O 4 and Pr-doped tetragonal ZrO 2 The Zr-Pr-O composite oxide cobalt-based catalyst, namely CDUT-CZP-II catalyst; the molar composition of the catalyst is (PrO 1.5 ) 0.12 (ZrO 2 ) 0.53 (CoO 1.5 ) 0.2 , the weight percent composition is: praseodymium ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com