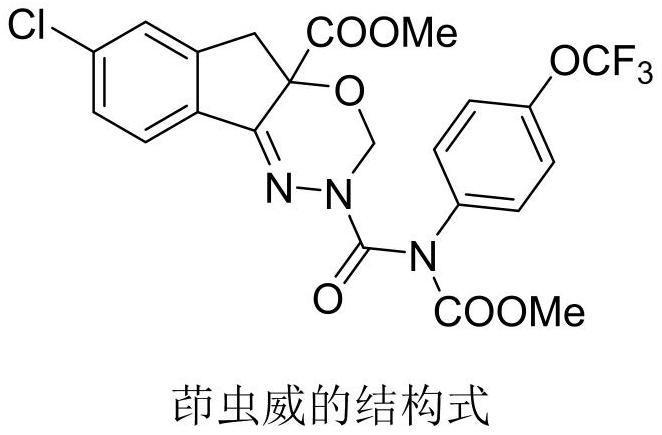
Improved process method for preparing 5-chloroindanone
A chlorindanone and process improvement technology, applied in the field of organic synthesis, can solve the problems such as no public refining method, and achieve the effects of shortening production hours, avoiding losses, and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 (traditional process)
[0037]
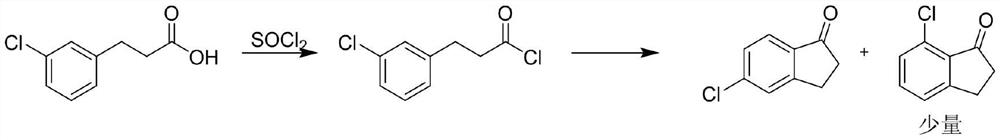
[0038] Put 225g (2.0mol, 1eq) of chlorobenzene into the reaction bottle, cool down to 25-30°C, add 333g (2.5mol, 1.25eq) of aluminum trichloride under stirring, and slowly add dropwise at the temperature of 25-30°C 254g (2.0mol, 1eq) of 3-chloropropionyl chloride, after the dropwise addition, keep warm for 3 hours. Add 466.7g (3.5mol, 1.75eq) of aluminum trichloride, 163g of sodium chloride and 23.3g of potassium chloride in molten state, and heat to 135°C for 4h. Sampling HPLC detects that there is no intermediate residue, the reaction solution is added to 3700g of ice water for hydrolysis, filtered, and dried to obtain 330g of black solid, which is dissolved in 1000mL of methanol and refluxed, filtered hot, and the filtrate is reheated to reflux, 30g of activated carbon is added, filtered hot, and the filtrate The temperature was lowered and crystallized to obtain 242.9 g of a yellow solid with a yield of 72.9% and HPL...
Embodiment 2
[0039] Embodiment 2 (traditional process)
[0040]
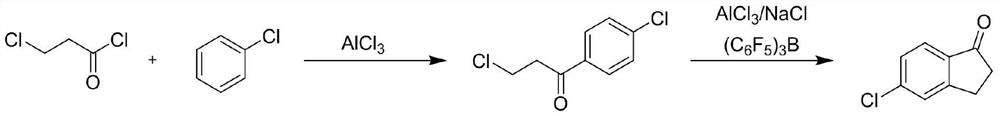
[0041] Put 227.4g (2.02mol, 1.01eq) of chlorobenzene into the reaction bottle, cool down to 25-30°C, add 314.7g (2.36mol, 1.18eq) of aluminum trichloride under stirring, and after stirring for 10 minutes, control the dropwise temperature Slowly add 254g (2.0mol, 1eq) of 3-chloropropionyl chloride dropwise at 25-45°C, and heat to 70-80°C for 2 hours after the addition is complete. Sampling GC detects that 0.5% of the raw material 3-chloropropionyl chloride remains. Add 514.7g (3.86mol, 1.93eq) of aluminum trichloride and 57.3g (0.98mol, 0.49eq) of sodium chloride in molten state, and heat to 150-165°C for 3h. Sampling was performed by HPLC to detect that no intermediate remained. After the heat preservation was completed, the temperature was lowered to 105-110°C. The reaction solution was added to 3700g of ice water for hydrolysis, filtered, and dried to obtain 330g of a black solid. The crude product 330g was divided into ...
Embodiment 3
[0043]
[0044] Put 227.4g (2.02mol, 1.01eq) of chlorobenzene into the reaction bottle, cool down to 25-30°C, add 314.7g (2.36mol, 1.18eq) of aluminum trichloride under stirring, and after stirring for 10 minutes, control the dropwise temperature Slowly add 254g (2.0mol, 1eq) of 3-chloropropionyl chloride dropwise at 25-45°C, and heat to 70-80°C for 2 hours after the addition is complete. Sampling GC detects that 0.5% of the raw material 3-chloropropionyl chloride remains. Add 514.7g (3.86mol, 1.93eq) of aluminum trichloride and 57.3g (0.98mol, 0.49eq) of sodium chloride in molten state, and heat to 150-165°C for 3h. Sampling was performed by HPLC to detect that there was no residual intermediate, and after the heat preservation was completed, the temperature was lowered to 105-110° C., and 2000 g of 4% hydrochloric acid prepared in advance was added dropwise. Control the dropping temperature at 90-95°C, stir for 10 minutes, let stand for 30 minutes, and separate the lower...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com