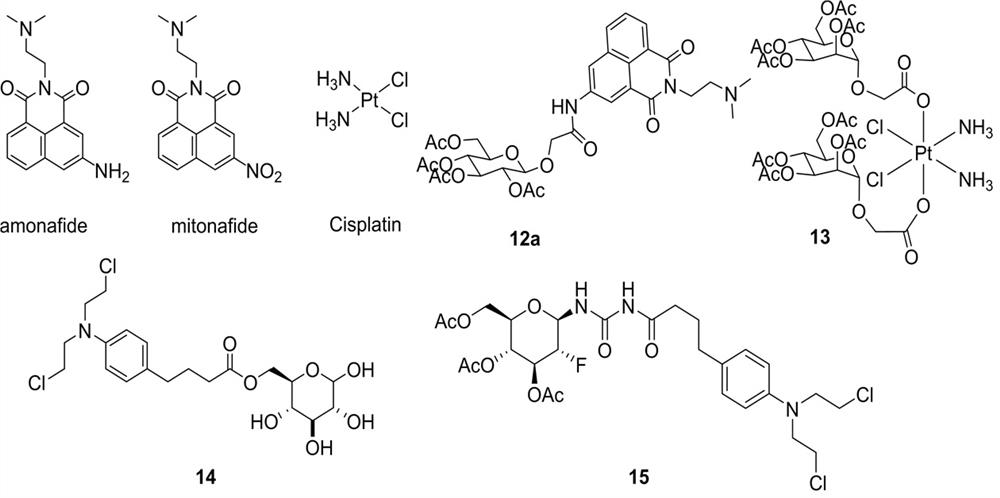
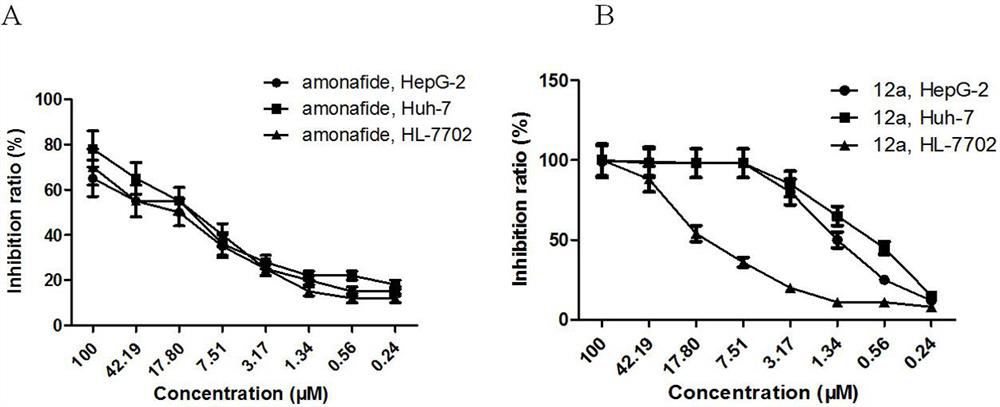
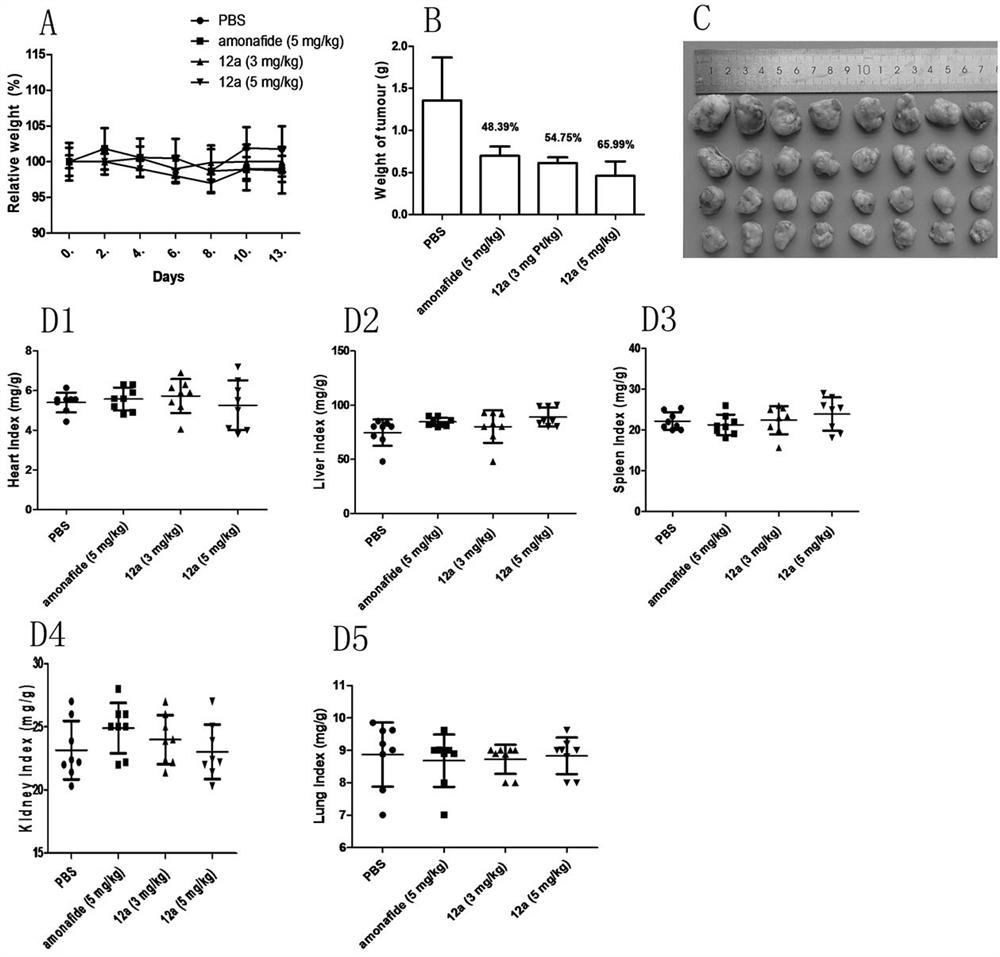
Glycosyl modified naphthalimide-polyamine conjugate as well as preparation method and application thereof
A technology of naphthalimide and sugar modification, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., to achieve the effects of improving water solubility and fat solubility, reducing toxic side effects, and improving maximum tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] Preparation of Compound 8a, 8b:
[0056] Compound 1 (glucose) reacts fully in the presence of acetic anhydride and sodium acetate to obtain compound 2 (fully acetylated glucose), and compound 2 reacts with a mixed solvent of THF / water under conditions of ammonia gas to obtain 1 The deprotected compound 3, after the compound 3 passed through the trichloroacetonitrile donor at the 1 position, and then reacted with 5a(5b) through glycosylation coupling reaction, the compound 6a(6b) was obtained, and the compound 6a(6b) was obtained under hydrogen atmosphere Compound 7a (7b) was obtained by debenzylation at 1-position. Compound 7a (7b) was catalyzed by DMF under the action of oxalyl chloride and reacted at room temperature to obtain compound 8a (8b).
[0057] The specific preparation process of compound 8a (8b) and compound 11 is not the invention point of the present invention, so it will not be described again.
[0058] Specific synthetic methods and process parameters o...
Embodiment 1
[0060] Example 1: 12a
[0061]
[0062] 1 H NMR (300MHz, Chloroform-d) δ9.06(s,1H),8.54(s,1H),8.36(d,J=12.3Hz,1H),7.96(s,1H),7.58(s,1H) ,5.32–4.96(m,4H),4.20(d,J=28.8Hz,3H),3.80(s,2H),3.36(s,2H),2.83(s,6H),2.00(s,12H). 13 C NMR(75MHz,Chloroform-d)δ170.58,170.20,170.01,169.41,167.22,164.09,163.62,136.32,133.98,130.00,127.35,124.06,121.80,100.66,72.15,72.02,71.54,68.39,68.12,61.61,55.25 ,43.64,35.42,20.95,20.68,20.56.ESI-MS(positive ion mode):m / z[M+H] + :calcd:672.2405; obsd:672.2407.Calcdfor C 32 h 37 N 3 o 13 : C 57.22%, H 5.55%, N 6.26%. Found: C 57.20%, H 5.50%, N 6.24%.
[0063] Its preparation method is as follows:
[0064] Compound 11 (1 mmol) and compound 8a (1.5 mmol) were ice-bathed in anhydrous acetone (5 mL) for half an hour, then reacted overnight at room temperature to obtain the target compound 12a.
[0065] Concrete synthetic route is as follows:
[0066]
Embodiment 2
[0067] Example 2: 12b
[0068]
[0069] 1 H NMR (300MHz, Chloroform-d) δ8.89(s, 1H), 8.33(d, J=7.0Hz, 1H), 8.15(s, 1H), 7.96(d, J=8.1Hz, 1H), 7.64 (s,1H),5.19(t,J=9.3Hz,1H),5.11–4.92(m,2H),4.54(t,J=6.5Hz,1H),4.31(d,J=5.8Hz,2H) ,4.21(s,2H),3.66(d,J=27.8Hz,2H),3.32(s,2H),2.87–2.73(m,2H),2.47(d,J=27.1Hz,6H),2.03( dt,J=13.0,8.2Hz,12H),1.23(s,2H). 13C NMR(75MHz,Chloroform-d)δ171.84,170.87,170.20,169.62,169.39,164.26,163.69,133.72,132.33,129.53,127.30,123.72,122.85,122.07,121.48,100.92,72.72,71.90,71.42,69.33,68.41 ,61.80,57.29,45.62,37.70,33.66,25.41,20.80,20.74,20.58.ESI-MS(positive ion mode):m / z[M+H] + :calcd:700.2718; obsd:700.2715.Calcdfor C 34 h 41 N 3 o 13 : C 58.36%, H 5.91%, N 6.01%. Found: C 58.26%, H 5.87%, N 5.98%.
[0070] Its preparation method is as follows:
[0071] The difference between the preparation method of compound 8b in Example 2 and Example 1 is that compound 6a is replaced by 5a for the reaction to finally obtain compound 8b.
[0072] Compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com