Synthesis method of highly ordered dendronized heterogeneous glycopolymer containing various glycosyl groups
A highly ordered, synthetic method of technology, applied in the field of synthesis of dendritic heterogeneous sugar-containing polymers, can solve the problem of inability to accurately simulate the strong recognition ability of natural polysaccharides, and cannot meet the needs and functions of sugar-containing polymers for diversification and functionalization. problems such as difficulty in obtaining high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 1. Synthesis of Compound 1
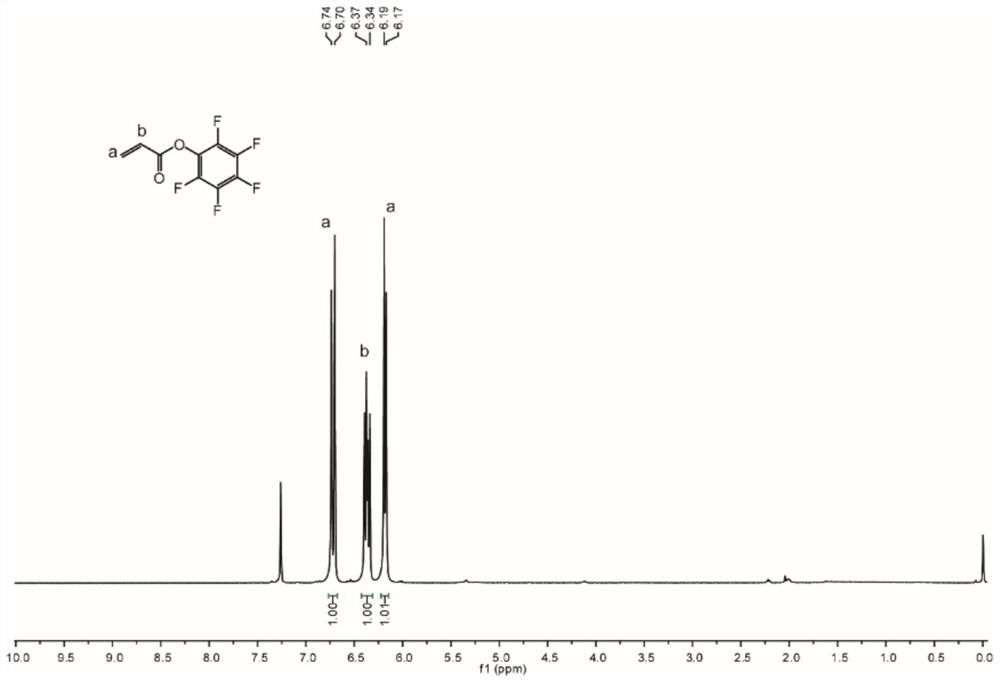
[0071]Heavy vapor triethylamine (0.6 mL, 4.0 mmol) was added dropwise to pentafluorophenol (0.62 g, 3.37 mmol) in anhydrous DCM solution (6 mL), and acryloyl chloride was added dropwise after stirring 15 min. (0.3 ml, 4.0 mmol), continuously stirred at 0 ° C for 30 min, and the reaction was at room temperature for 2 h. After completion of the reaction, the reaction solution was diluted with DCM, filtered, DCM washing filter, and the filtrate was washed twice with saturated brine. The organic phase was dried over anhydrous sodium sulfate, filtered, spurly removed the solvent, and obtained a colorless oil product PFPA (1,0.6 g, yield 70%) was obtained by silica gel column chromatography (PE).
[0072] Refer to the nuclear magnetic hydrogen spectrum of the obtained compound 1 figure 1 .
[0073] 1 H NMR (500MHz, CDCL 3 : δ = 6.72 (D, J = 17.0 Hz, 1H), 6.37 (DD, J = 17.5, 10.5 Hz, 1H), 6.18 (D, J = 10.5 Hz, 1H). 19 F NMR (470MHz, CDCL 3 : δ = -152.5...
Embodiment 2
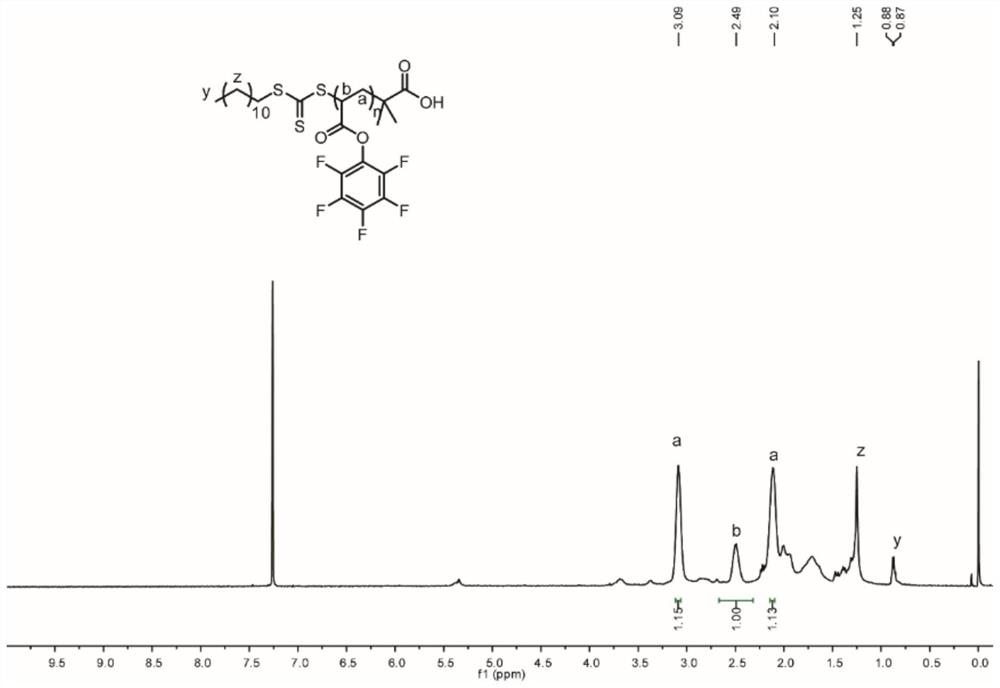
[0079] 1. Synthesis of Compound 1 (PFPA) and Compound 2 (PPFPA) is as in Example 1
[0080] 2. Preparation of sugar polymer P1-P8
[0081] In a nitrogen-filled glove box, Compound 2 (50 mg, 0.21 mmol), and branned glycosamine (S1-S8, 0.10 mmol), DMAP (12.8 mg, 0.10 mmol) were added to the glass bottle, then add anhydrous DMF (4 mL) dissolved, the mixture was stirred at 65 ° C for stirring at a glove box filled with nitrogen 24 h. After completion, the reaction liquid was subjected to diethyl ether three times, centrifuged, and dried overnight at 40 ° C vacuum to give a sugar-containing polymer protected by brown powder OAC (corresponding to P1-P8 according to S1-S8), yield at 55% - Between 58%.
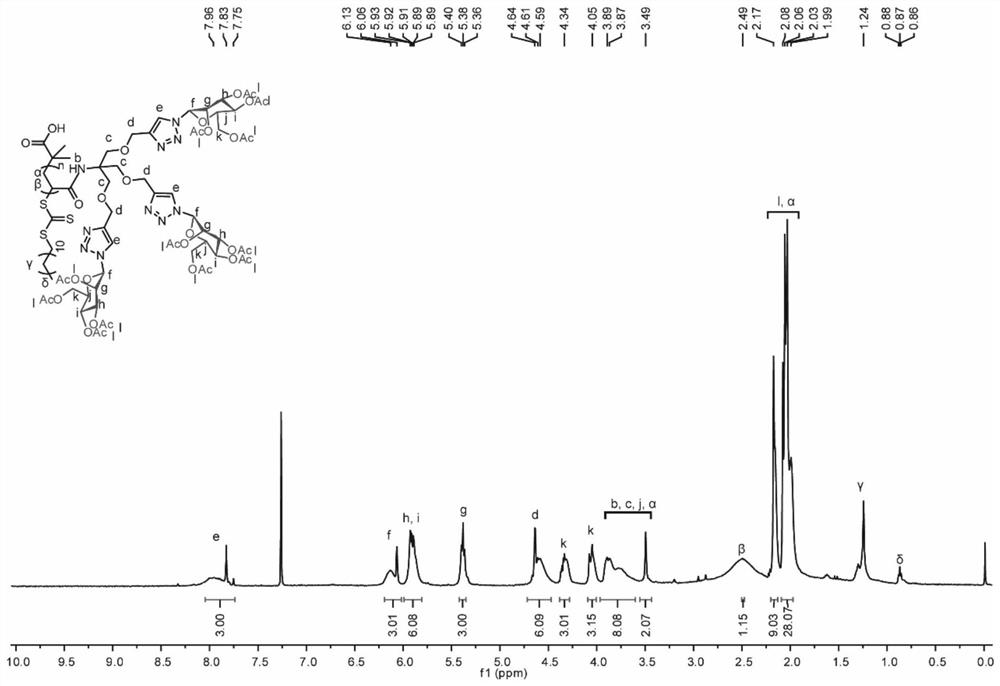
[0082] The prepared sugar-containing polymer P1-P8 nuclear magnetic hydrogen spectrum is respectively Figure 3 to 10 Indicated.
[0083] 3. Preparation of branched sugar-containing polymer P9-P16
[0084] At room temperature, Meona (0.48 mmol) was added dropwise to the sugar-containing po...
Embodiment 3
[0089] 1. Synthesis of Compound 1 (PFPA) and Compound 2 (PPFPA) is as in Example 1
[0090] 2. Preparation of sugar-containing polymer P1-P8 and P9-P16 is as in Example 2
[0091] 3. Turbidity method Test the specific identification effect of branches containing sugar-containing polymer P9-P16 and knife bean protein A (con a)
[0092] The CON A and the sugar polymer were dissolved in the HEPES buffer of pH = 7.4. First, 238 mgHepes is dissolved in 10 ml of deionized water, and the pH = 7 was adjusted with NaOH, and then 62.5 mg MnCl 2 , 55.5mg CaCl 2 480 mg NaCl was dissolved with 15 ml of deionized water and added to the HEPES aqueous solution to adjust the pH, and 100 ml of volumetric flask was added, filtered with the filtration membrane before using the filter membrane to prepare 0.01 m HEPES buffer. Then, 1 mg / ml of CON A solution was configured with a buffer, and 1 mg / ml of sugar-containing polymer solution. After adding 0.40 ml of CON A solution (1 mg / ml), after addin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com