Julolidine compounds, and preparation method and application thereof
A technology of julolidine and compound is applied in the field of preparation of dye sensitizers, which can solve the problems of narrow absorption, wide absorption range and difficulty in the visible light region, and achieve the effect of good photoelectric conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
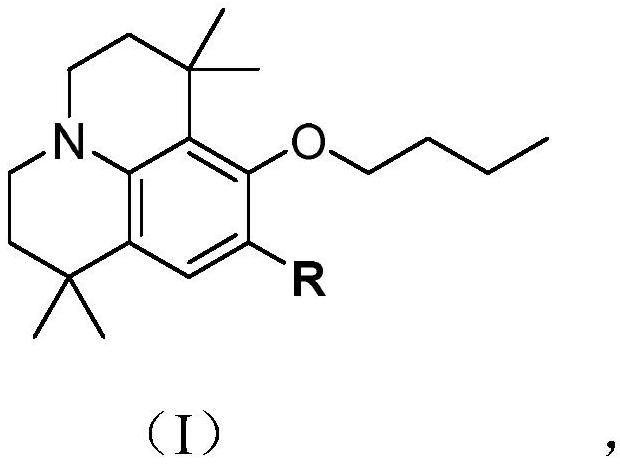
[0025] The reaction formula of preparing the julolidine compounds shown in formula (I-1) is as follows:
[0026]
[0027] Preparation of julolidine compounds as shown in formula (I-1), comprises the following steps:
[0028] 1) Preparation of Vilsmeier reagent: under a nitrogen atmosphere, extract 5mL of anhydrous DMF into a Shrek tube, slowly add POCl dropwise in an ice bath 3 (0.5mL, 5mmol), stirred for 1h, and the preparation of the reagent was completed.
[0029] 2) Preparation of compound II: Weigh compound III (500mg, 1.7mmol) and dissolve it in 1,2-dichloroethane (5mL), inject it into the Shrek tube in step 1), and react at 90°C for 6h, and the reaction is complete Finally, the reaction solution was washed with water, extracted with ethyl acetate, and the organic layer was dried over anhydrous sodium sulfate. After removing the solvent, the residue was purified by column chromatography (VPE:VEA=10:1) to obtain compound II, 320.0 mg of yellow powder solid , yield 59...
Embodiment 2
[0034] The reaction formula of preparing the julolidine compounds shown in formula (I-2) is as follows:
[0035]
[0036] Preparation of julolidine compounds as shown in formula (I-2), comprises the following steps:
[0037] Preparation of compound I-2: weigh compound II (128.7mg, 0.39mmol), rhodanine-3-acetic acid (224.1mg, 1.17mmol), dissolve in acetonitrile (16mL) and chloroform (8mL) and transfer to Shrek Tube, piperidine (0.3mL) was extracted and added dropwise, heated at reflux at 60°C for 12h under a nitrogen atmosphere, the reaction solution was cooled to room temperature, the solvent was removed, and the residue was passed through column chromatography (V DCM :V MeOH :V HOAc = 100:1:2) to obtain compound I-2, 137.1mg of red powder solid, yield 70%, melting point: 216-217°C.
[0038] The compound of the structure shown in the obtained formula (I-2) is carried out nuclear magnetic spectrum analysis, and the results are as follows: 1H NMR (500MHz, DMSO) δ8.01(s, 1...
Embodiment 3
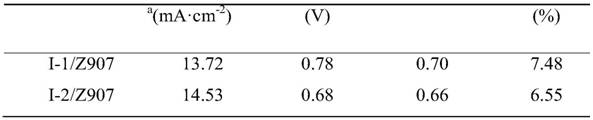
[0039] Example 3 Julonidine compounds are used as co-sensitizers for ruthenium metal dyes:
[0040] Firstly, mix the julolidine compounds and ruthenium metal dye Z907 prepared in Examples 1-2 at a material ratio of 1:1, and dissolve them in CHCl with a volume ratio of 10:1. 3 In mixed solution with methanol, prepare dye sensitizer solution (concentration of compound is 3×10 -4 mol L -1 ).
[0041] Bilayer TiO prepared by screen printing 2 The nanoparticle film is used as a photoelectrode, including the following steps:
[0042] 1) First print a layer of 20nm TiO with a thickness of 12μm on the conductive glass FTO 2 Particles were calcined in a muffle furnace at 450 °C for 30 min, and then immersed in 0.04 mol L -1 Concentration of TiCl 4 Pretreatment in aqueous solution at 70 °C for 30 min to prepare double-layer TiO 2 Nanoparticle film. Then remove the bilayer TiO 2 The nanoparticle film was rinsed with water and ethanol, respectively, and dried with a hair dryer. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com