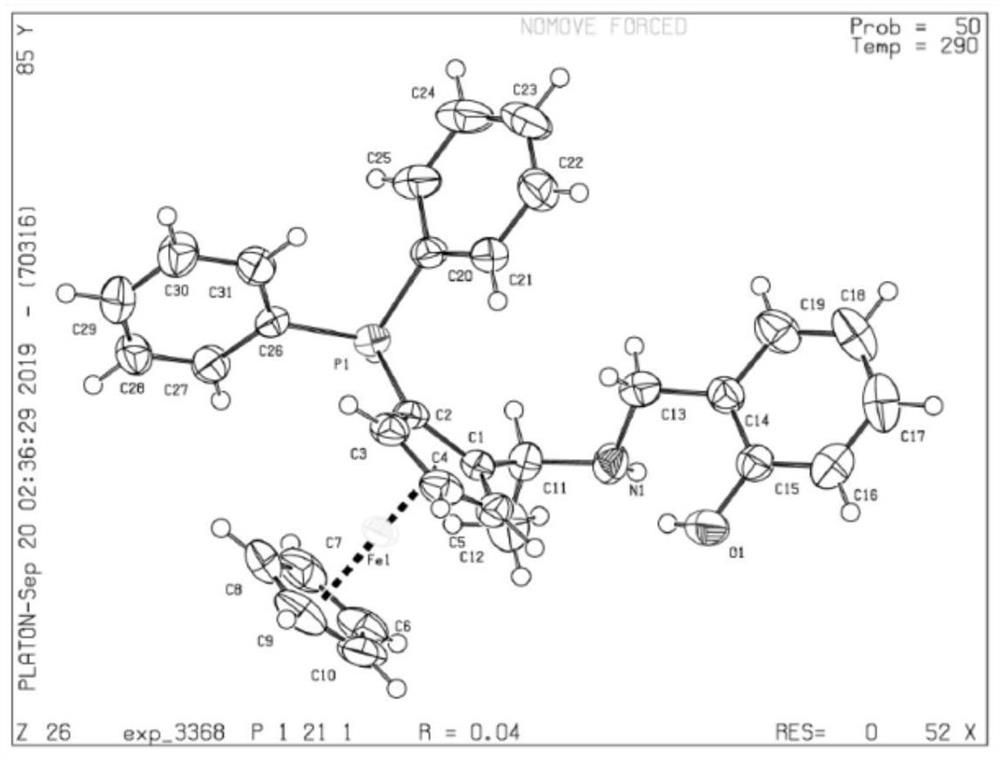
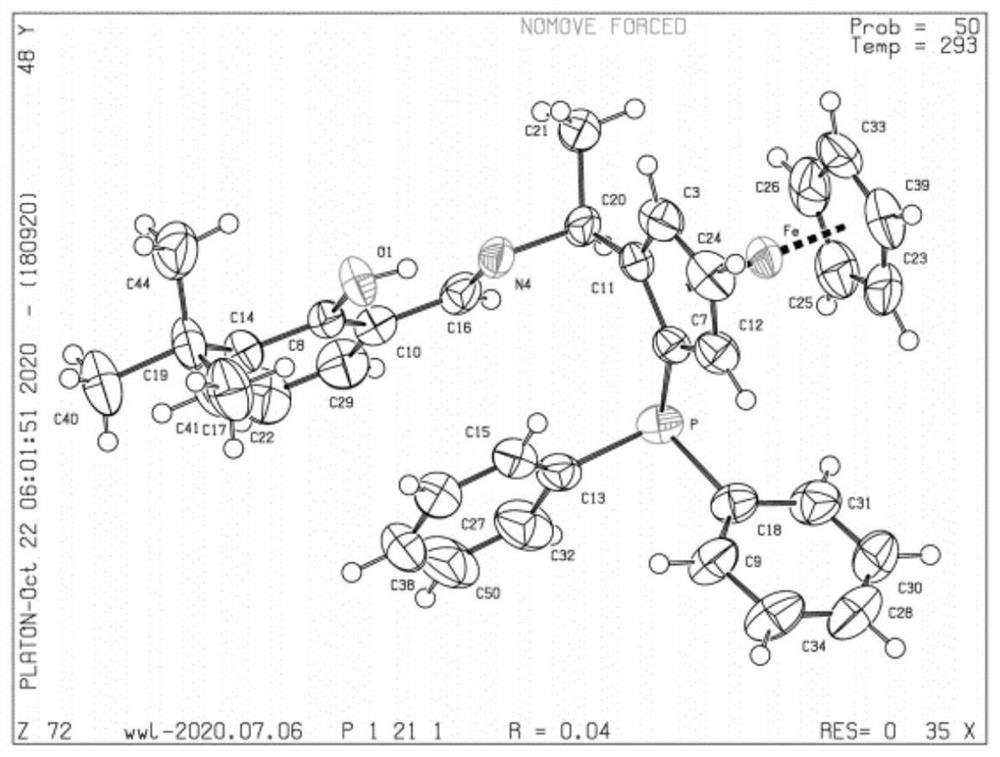
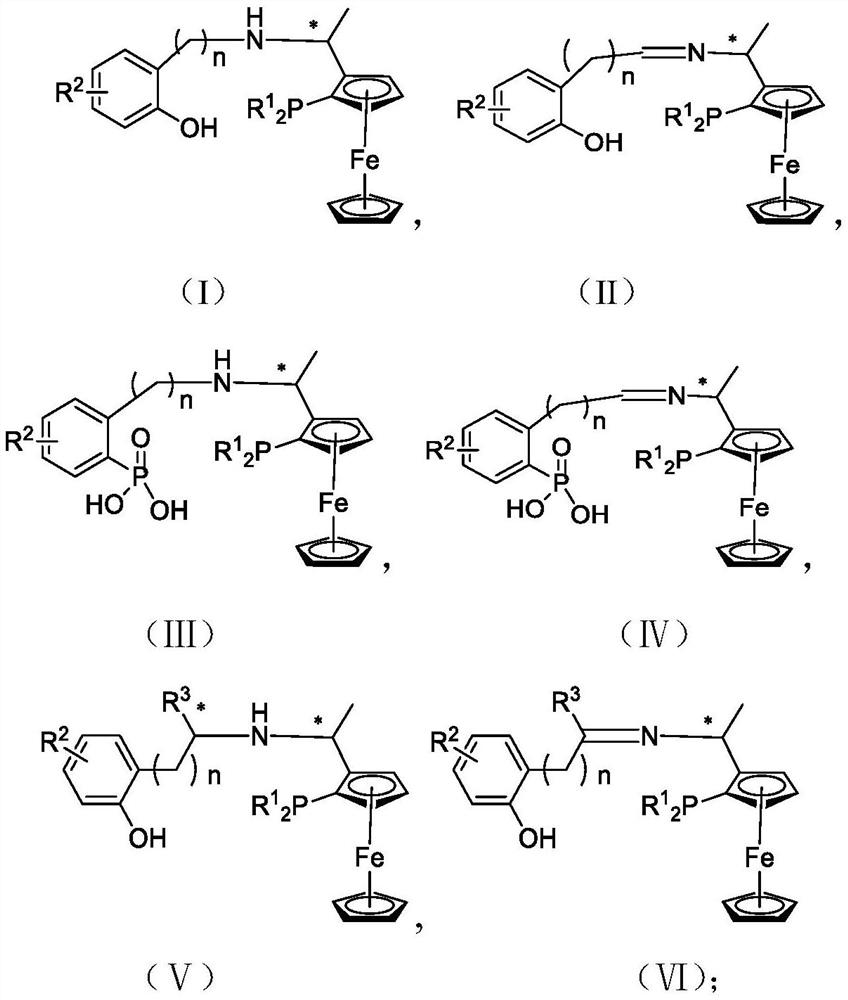
PNO ligand containing planar chiral ferrocene and application thereof
A chiral ferrocene and ligand technology, which is applied in the direction of metallocene, catalytic reaction, formation/introduction of hydroxyl groups, etc., can solve the problems of limited practical application value, poor ligand stability, expensive catalyst raw materials, etc., and achieve a large Large-scale production, easy synthesis, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 contains the ligand of face chiral ferrocene (S FC )-L1 synthesis
[0053]
[0054] N 2 Under protection, a solution of n-butyllithium in n-hexane (12.4mL, 1.4M) was added dropwise to a solution of (S)-1 (3.6g, 14mmol) in anhydrous ether (20mL), and the reaction solution was kept at 0°C, and The dropping time was controlled for about 20 minutes to complete the dropping, and the reaction solution was stirred at room temperature for 1.5 hours, and then diphenylphosphine chloride (6.2 g, 28 mmol) in 10 mL of diethyl ether solution was slowly added dropwise to the reaction system. After the dropwise addition was completed, it was refluxed for 4h. Cool the reaction solution to room temperature, put it in an ice-water bath, slowly add a saturated aqueous solution of sodium bicarbonate dropwise, and extract the orange-yellow product with ether, combine the organic phases, wash with water, dry over anhydrous sodium sulfate, and dry in vacuo to obtain an orange...
Embodiment 2
[0062] Embodiment 2 contains the ligand of chiral ferrocene (S FC )-L8 synthesis
[0063]
[0064] Similar to the synthesis method of Example 1, at room temperature, under the protection of nitrogen, the synthesized compound (S,R)-4 (2.0g, 4.8mmol) was dissolved in absolute ethanol (15mL), and after being stirred to a homogeneous phase, the 2-Hydroxy-1-naphthaldehyde (0.91g, 5.3mmol) was added to the reaction system in batches, and the reaction was complete in about 6 hours. After the reaction was completed, an orange-red suspension was obtained. Then add NaBH to the suspension in batches at 0°C 4 Solid (0.46g, 12mmol), after the addition was completed, the reaction solution was raised to room temperature and stirred for 2h. After the reaction was monitored by TLC, it was quenched with water, extracted three times with DCM, and the organic phase was combined. The organic phase was washed with saturated brine, and finally washed with anhydrous Dry over sodium sulfate, remo...
Embodiment 3
[0065] Embodiment 3 contains the ligand of chiral ferrocene (S FC )-L15 synthesis
[0066]
[0067] Similar to the synthesis method of Example 1, at room temperature, under the protection of nitrogen, the synthesized compound (S,R)-4 (2.0g, 4.8mmol) was dissolved in absolute ethanol (15mL), and after being stirred to a homogeneous phase, the 3-tert-butyl salicylaldehyde (0.94g, 5.3mmol) was added to the reaction system in batches, and the reaction was complete in about 6 hours. After the reaction was completed, an orange-red suspension was obtained. Then add NaBH to the suspension in batches at 0°C 4 Solid (0.46g, 12mmol), after the addition was completed, the reaction solution was raised to room temperature and stirred for 2h. After the reaction was monitored by TLC, it was quenched with water, extracted three times with DCM, and the organic phase was combined. The organic phase was washed with saturated brine, and finally washed with anhydrous Dry over sodium sulfate, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com