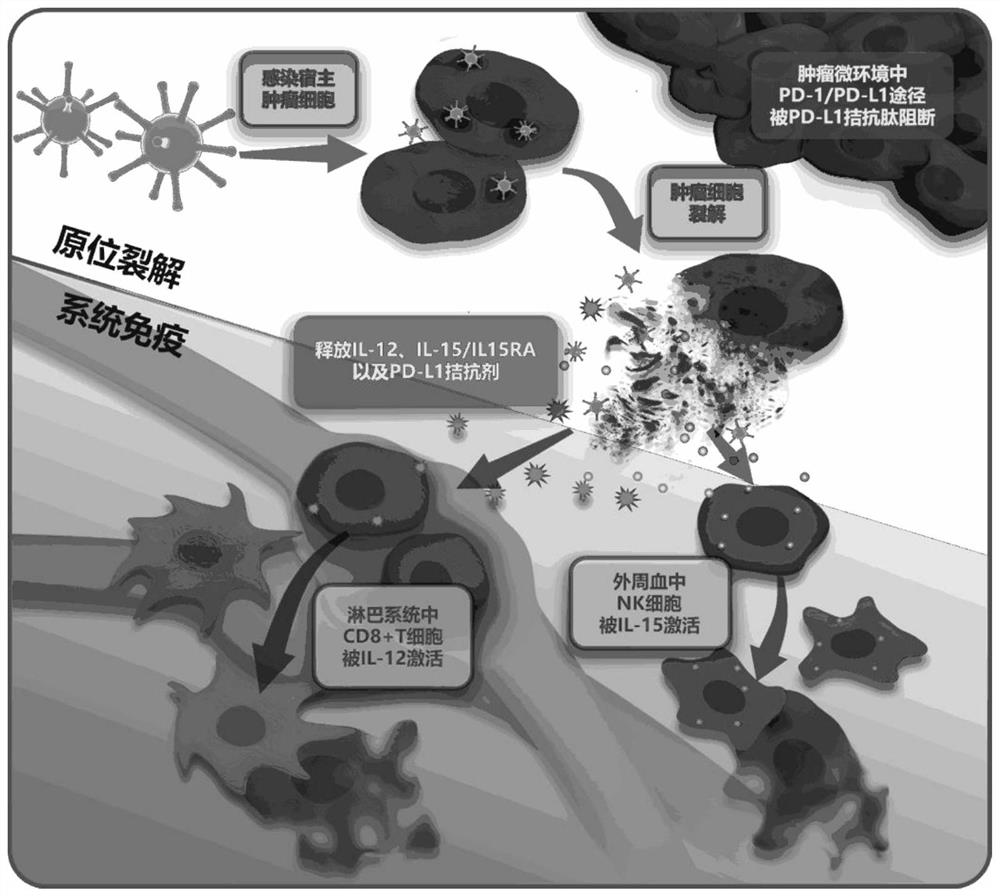
Use of oncolytic virus VG161 or combination of oncolytic virus VG161, gemcitabine and albumin paclitaxel in preparation of drugs for treating pancreatic cancer
A technology of VG161 and oncolytic virus, which is applied in the field of combination of gemcitabine and albumin-paclitaxel in the preparation of drugs for the treatment of pancreatic cancer, can solve the problems such as the application of oncolytic virus that has not been reported in the literature, and achieves increased therapeutic effect, less toxic and side effects, and pharmacological effects. clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1VG161
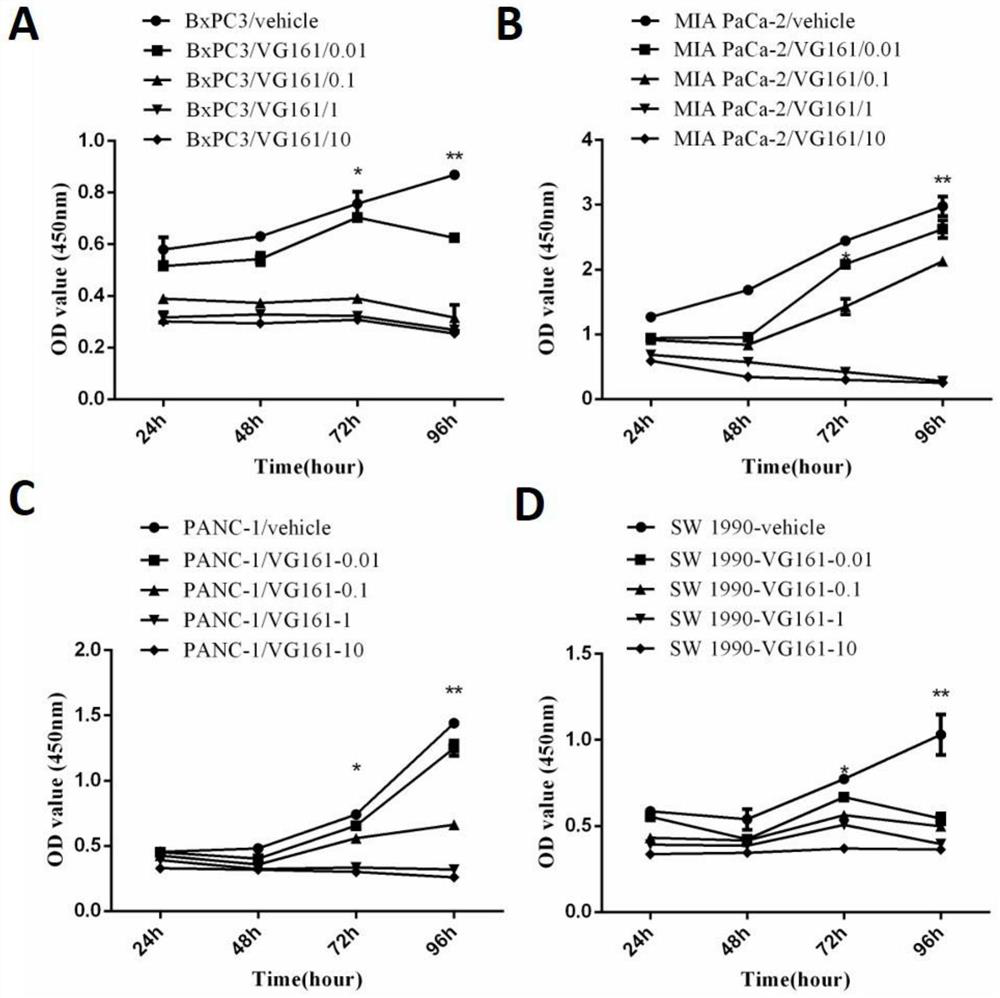
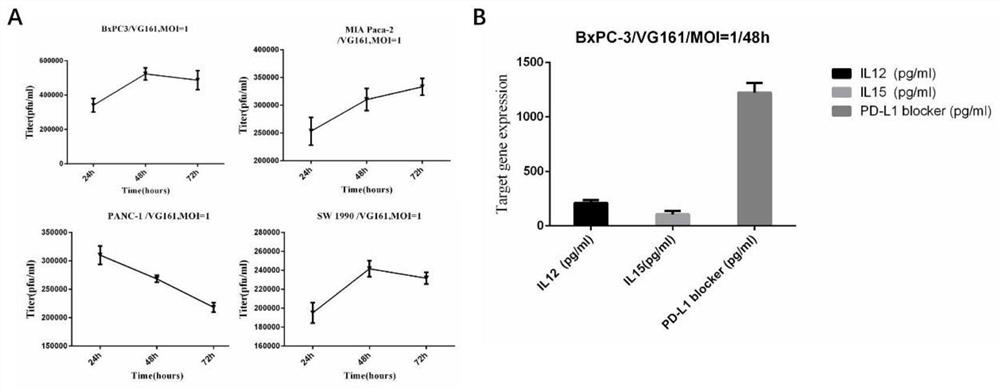
[0036] Example 1 VG161 effectively kills pancreatic cancer cells in vitro and promotes apoptosis
[0037] 1. Cell killing and apoptosis-promoting experiments in vitro
[0038] In vitro experiments confirmed that VG161 single drug can kill and promote apoptosis of pancreatic cancer cell lines, which was detected by CCK8 and cell flow assay.
[0039] CCK8 is Cell Counting Kit-8, which can be used for easy and accurate cell proliferation and toxicity analysis. The basic principle is: the reagent contains WST-8 [chemical name: 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4 -disulfonic acid benzene)-2H-tetrazole monosodium salt], it is in the cell under the effect of electron carrier 1-methoxy-5-methylphenazinium sulfate dimethyl sulfate (1-Methoxy PMS) The dehydrogenase is reduced to a highly water-soluble yellow formazan product (Formazan dye). The amount of formazan produced is directly proportional to the number of living cells. This feature can therefore be used dire...
Embodiment 2VG16
[0052] Example 2 VG161 effectively kills pancreatic cancer cells in vivo
[0053] In vivo pharmacodynamic research is an important process to verify the efficacy of drugs. By constructing a pancreatic cancer subcutaneous tumor model on nude mice and injecting it into the tumor, the therapeutic effect of VG161 in solid tumors is verified.
[0054] Inject BxPC3 cell suspension in the right armpit of nude mice, and tumors will form after 2 weeks, until the size of the tumor reaches 800mm 3 Intratumoral injection of VG161 was performed, and the vehicle was used as a control. Observation indicators include tumor volume change and mouse body weight, which are measured every 3 days, and the survival curve of mice is recorded. Observation endpoints include mouse death or mouse tumor volume exceeding 2000mm 3 .
[0055] In order to explore the optimal concentration of VG161 combined with other drugs, five consecutive and single injections of VG161 at different concentrations were pe...
Embodiment 3
[0059] Example 3 VG161 combined with gemcitabine and nab-paclitaxel
[0060] After obtaining the optimal combination concentration of VG161 based on Example 2, the BxPC3 nude mouse model was established again to explore the therapeutic effect of VG161 combined with the first-line chemotherapy regimen for pancreatic cancer (GEM+Nab-PTX regimen).
[0061] The GEM+Nab-PTX regimen, that is, gemcitabine combined with nab-paclitaxel, is currently the commonly used first-line neoadjuvant chemotherapy regimen for pancreatic cancer. Many studies have confirmed that the combination of the two drugs is beneficial to prolong the survival time of patients with pancreatic cancer. Vehicle was set as the negative control group, and VG161 single drug and GEM+Nab-PTX regimen were set as the positive control group to explore the therapeutic effect of the combined regimen of VG161+GEM+Nab-PTX on pancreatic cancer. At the same time, the administration of VG161 first followed by the GEM+Nab-PTX re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com