A kind of preparation and use method of quality control strain quantitative pellet
A technology of small balls and strains, which is applied in the field of preparation of quantitative small balls for quality control strains, can solve the problems of heavy workload and cumbersome operation, and achieve the effects of low cost, simple process and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A method for preparing quantitative pellets of quality control strains, comprising the following steps:
[0023] (1) The single colony of the quality control strain is picked into the liquid medium, cultivated to the logarithmic growth phase, and the bacterial liquid is obtained;
[0024] (2) take quantitative bacterial liquid to be diluted in freeze-dried bacterial strain protective agent to obtain protective agent bacterial liquid;
[0025] (3) take a quantitative protective agent bacterial liquid, and pre-freeze it after forming into a ball with liquid nitrogen;
[0026] (4) vacuum freeze-drying the pre-frozen pellets obtained in step (3) to obtain dry pellets;
[0027] (5) Packed into sterile vials, each sterile vial is equipped with a dry pellet.
[0028] In step (2), each 100ml of the freeze-dried bacterial strain protection agent includes the following components and their mass ratios: 10% protein, 5% water-soluble sugar, 10% freeze-dried plasticizer, and the b...
Embodiment
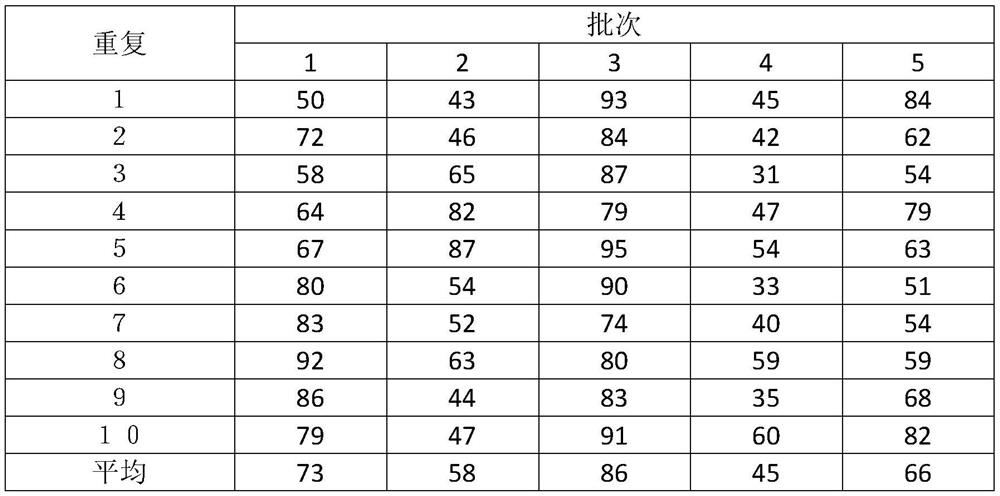
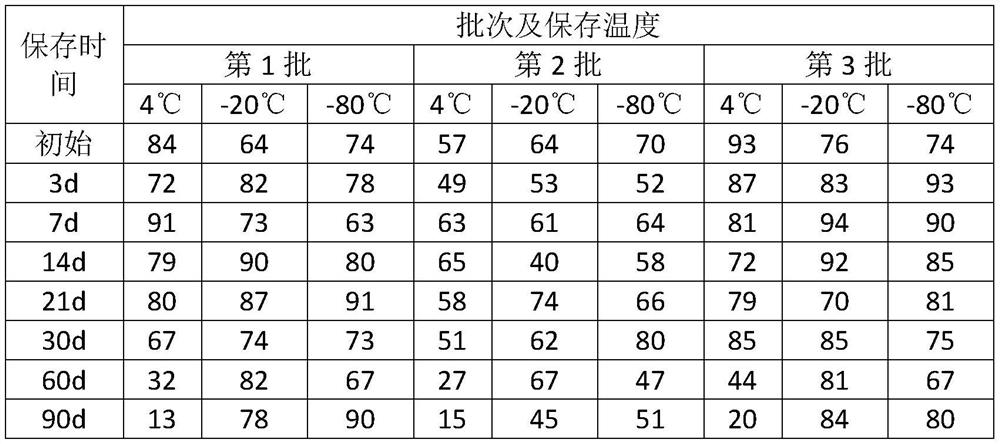
[0039] (1) The single colony of Escherichia coli of the quality control strain was picked into the tryptone soy peptone broth and cultivated to the logarithmic growth phase, that is, culturing at 36 degrees for 18-24 hours, to obtain a high-purity bacterial liquid with better activity;
[0040] (2) The freeze-dried strain protection agent includes the following components and their mass ratios: protein 10%, water-soluble sugar 5%, freeze-dried plasticizer 10%, the balance is water, and each 100ml freeze-dried strain protectant Including protein 10g, water-soluble sugar 5g, freeze-dried plasticizer 10g, and the balance is water. After all components are fully dissolved, autoclave at 115°C for 20 minutes, take it out, and cool it for later use. Among them, protein includes calf serum 3.0g and skim milk powder 7.0g, water-soluble sugar includes sucrose 3.0g and trehalose 2.0g; freeze-dried plasticizer includes polyethylene glycol 4000 3.0g, polyethylene glycol 6000 4.0g and Poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com