Esterase response nano-drug as well as preparation method and application thereof
A nano-medicine and drug technology, applied in nano-medicine, nanotechnology, nanotechnology, etc., can solve the problems of poor targeting, toxic and side effects that cannot be ignored, and difficulty in achieving satisfactory therapeutic effects, so as to achieve low toxic and side effects and improve bioavailability , the effect of good clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] In this example, nanomedicine was prepared by the following method:
[0061] Esterification: (1) Dissolve VGB polypeptide (20mg), 2,4,6-trichlorobenzoyl chloride (60ul), 4-dimethylaminopyridine, and triethylamine in 1ml of dimethylformamide, avoiding Light reaction for 30 minutes to obtain a homogeneous solution;
[0062] (2) Dissolve 8.8 mg of oridonin ORI in 1 ml of dimethylformamide, add it to the homogeneous solution in (1) above, react at room temperature overnight (time 15 hours), TLC tracking monitoring, The reaction is over.
[0063] Purification: (1) Add excess ether, let it stand for 30 minutes, and filter with suction to obtain a yellow crude product;
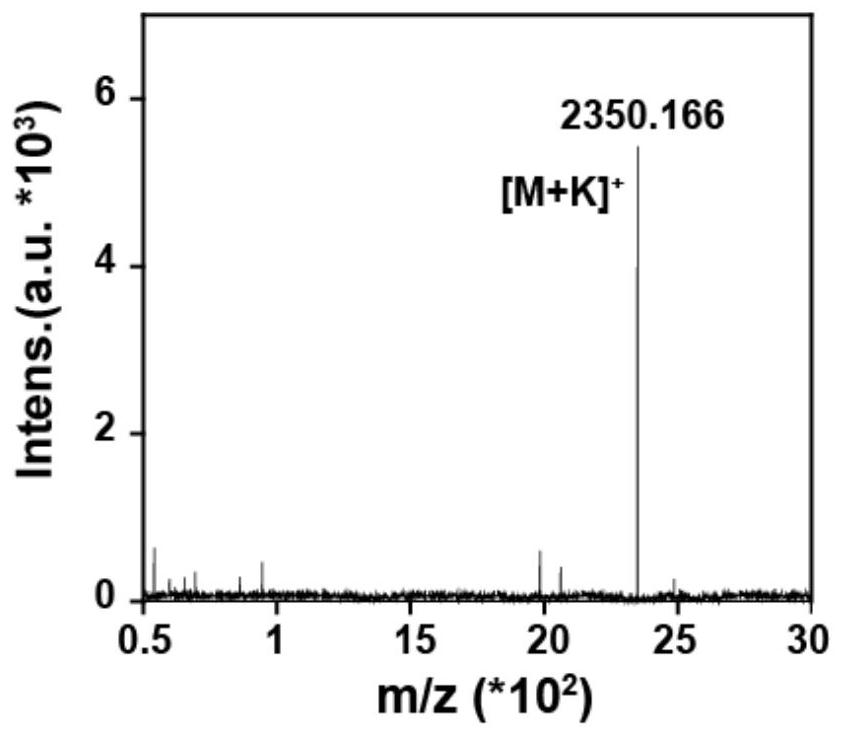
[0064] (2) Dissolve the yellow solid in the above (1) with isopropanol, recrystallize and purify to obtain the ORI-VGB amphiphilic molecule. The mass spectrum of the ORI-VGB amphiphilic molecule is as follows figure 1 As shown, it can be seen that the amphiphilic molecule was successfully constructed. fig...
Embodiment 2
[0067] The purpose of this example is to determine the changes in the morphology, particle size and potential of the nanomedicine in the esterase solution.
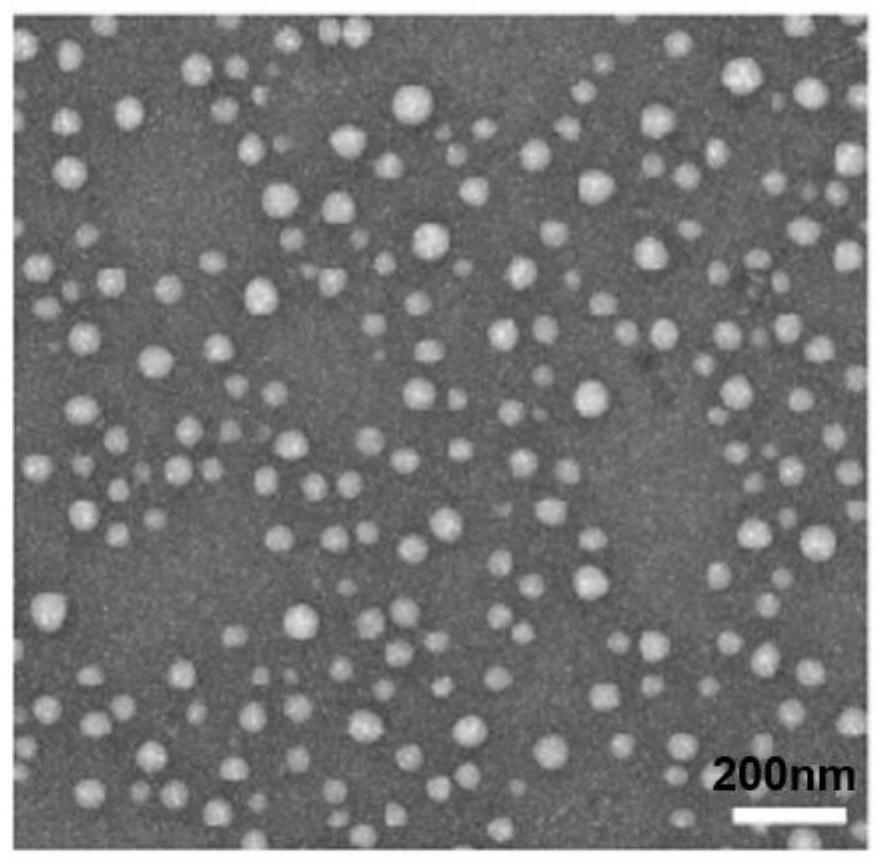
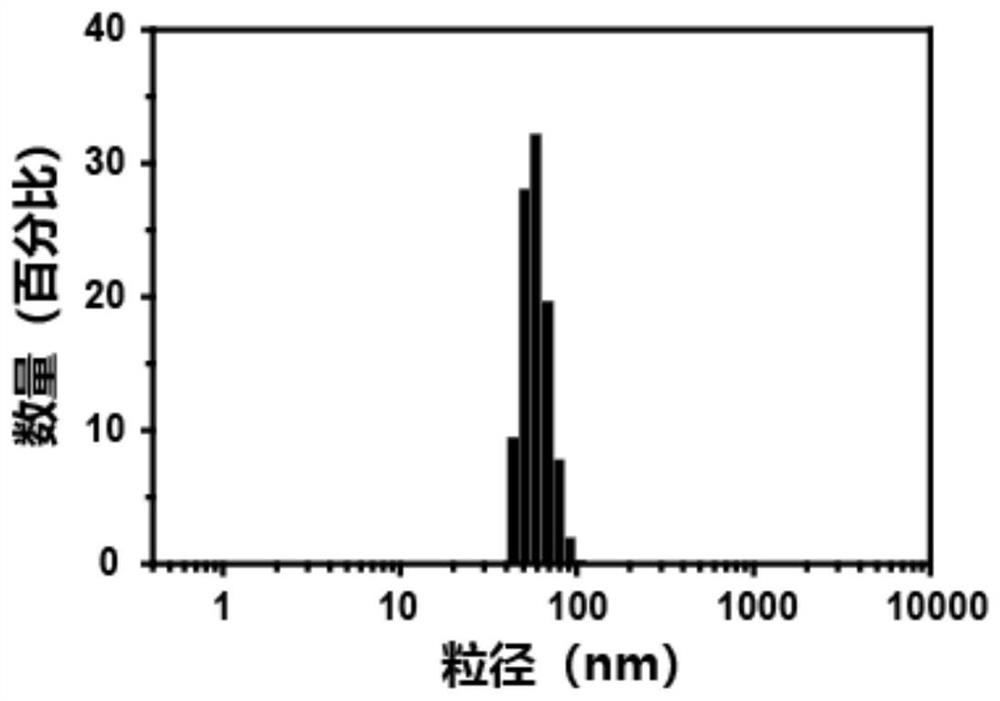
[0068] The nanomedicine obtained in Example 1 was dissolved in 1 ml of pig liver esterase (100 U / ml, PBS buffer) stock solution. Within a specified time interval, mix 20 μL of the incubation solution with 180 μL of acetonitrile to terminate the esterase reaction, centrifuge at 6000 rpm for 5 min, and finally observe the changes in morphology, particle size and potential. The result is as Figure 4 with Figure 5 shown. Figure 4 It is the transmission electron micrograph of the nano-medicine after being treated with esterase for 1 hour; from Figure 4 It can be seen that the nano-medicine has no obvious nano-spherical structure after esterase treatment; Figure 5 It is the particle size potential distribution diagram, after the esterase treatment, the particle size distribution of the nanomedicine changes rapidly from...
Embodiment 3
[0070] The purpose of this example is to verify the in vitro anti-tumor effect of the nanomedicine prepared in Example 1.
[0071] A non-functional VGC polypeptide (CHDQIHNKEQCPGI) in which the sequence of VGB polypeptides was scrambled was used as a control peptide, and ORI was linked with a VGC polypeptide according to the method in Example 1 as a control sample (abbreviated as ORI-VGC-NPs). Wherein, the amino acid sequence of the VGC polypeptide is shown in SEQ ID NO: 2, and its preparation method refers to the preparation of VGB above.
[0072] Firstly, the tumor cells EC109 were divided into 6×10 per well 3 seeded into 96-well plates and allowed to attach overnight. Cells were then treated with serially diluted experimental samples for 24 hours. The cell viability was assessed using the CCK-8 kit, and the results were as follows Image 6 shown. The VGB group had no obvious killing effect on the tumor, and the IC of the ORI and ORI-VGC-NPs treatment groups 50 were 67....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com