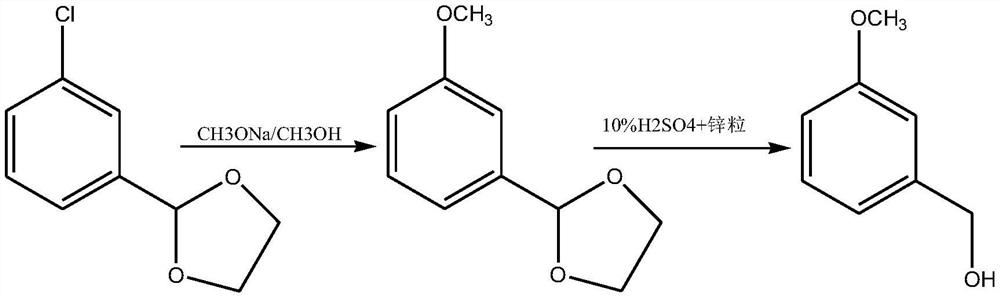
Synthesis method of m-methoxybenzyl alcohol
A technology of m-methoxybenzyl alcohol and methoxybenzyl alcohol is applied in the synthesis field of m-methoxybenzyl alcohol, and can solve the problem of unsafe synthesis process of m-methoxybenzyl alcohol, low product yield and complicated process and other problems, to achieve the effect of high product yield, cheap and easy-to-obtain raw materials, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of synthetic method of m-methoxybenzyl alcohol, comprises the steps:
[0029] One, the preparation of m-chlorobenzaldehyde condensed with ethylene glycol:
[0030] (1) Add m-chlorobenzaldehyde and toluene (solvent) in the reaction vessel, then add ethylene glycol and sulfuric acid and stir evenly, then heat to 100°C for condensation reaction for 7 hours, remove the toluene and ethylene glycol after the reaction , then rectifying, promptly obtains said ethylene glycol acetal m-chlorobenzaldehyde; The mol ratio between said m-chlorobenzaldehyde, ethylene glycol and said sulfuric acid in this step is 1:2:0.3;
[0031] Two, the preparation of ethylene glycol condensed m-methoxybenzaldehyde:
[0032] (1) Get 1mol of the gained ethylene glycol condensed m-chlorobenzaldehyde and dissolve it in the anhydrous methanol of 500mL and stir evenly to obtain a mixed solution;
[0033] (2) Under stirring conditions, add 1.2mol of sodium methoxide (condensing agent) to the mixe...
Embodiment 2
[0043] A kind of synthetic method of m-methoxybenzyl alcohol, comprises the steps:
[0044] One, the preparation of m-chlorobenzaldehyde condensed with ethylene glycol:
[0045] (1) Add m-chlorobenzaldehyde and xylene (solvent) in the reaction vessel, then add ethylene glycol and sulfuric acid and stir evenly, then heat to 120°C for condensation reaction for 5 hours, remove the toluene and ethylene glycol after the reaction Alcohol, rectifying then, promptly obtains described ethylene glycol acetal m-chlorobenzaldehyde; The mol ratio between described m-chlorobenzaldehyde, ethylene glycol and described sulfuric acid in this step is 1:3:0.1;
[0046] Two, the preparation of ethylene glycol condensed m-methoxybenzaldehyde:
[0047] (1) Get 0.5mol of the gained ethylene glycol condensed m-chlorobenzaldehyde and dissolve it in 500mL of anhydrous methanol and stir to obtain a mixed solution;
[0048] (2) Under stirring conditions, add 0.75mol of sodium methoxide (condensing agent...
Embodiment 3
[0056] A kind of synthetic method of m-methoxybenzyl alcohol, comprises the steps:
[0057] One, the preparation of m-chlorobenzaldehyde condensed with ethylene glycol:
[0058] (1) Add m-chlorobenzaldehyde and toluene (solvent) in the reaction vessel, then add ethylene glycol and sulfuric acid and stir evenly, then heat to 110°C for condensation reaction for 10 hours, remove the toluene and ethylene glycol after the reaction , then rectifying, promptly obtains said ethylene glycol acetal m-chlorobenzaldehyde; The mol ratio between said m-chlorobenzaldehyde, ethylene glycol and said sulfuric acid in this step is 1:5:0.5;
[0059] Two, the preparation of ethylene glycol condensed m-methoxybenzaldehyde:
[0060] (1) Get 0.8mol of gained ethylene glycol acetal m-chlorobenzaldehyde and be dissolved in the anhydrous methanol of 500mL and stir, obtain mixed solution;
[0061] (2) Under stirring conditions, add 0.8mol of sodium methoxide (condensing agent) to the mixed solution, he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com