Method for preparing alpha-hexylcinnamyl aldehyde
A technology of hexyl cinnamaldehyde and n-hexanal, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of high price, cumbersome separation and purification process, and low yield, so as to reduce self-condensation Effects of side reactions, improvement of cross-condensation selectivity, and prolongation of catalyst life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
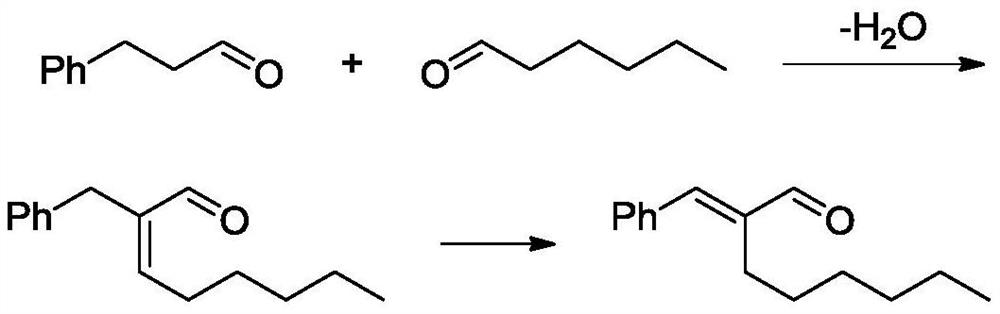
[0047] Proline catalyzes the condensation of phenylpropionaldehyde and n-hexanal to synthesize 2-benzyl 2-octenal.
[0048] At room temperature, a 2L three-necked flask equipped with a magnetic stirrer, temperature and reflux condenser was placed in an oil bath, and after the system was replaced 3 times with an oil pump and nitrogen, phenylpropanal (214.7g, 1.6mol ), n-hexanal (176.3g, 1.76mol) and solvent methyl tert-butyl ether (500mL). Turn on the oil bath and stir. After the raw materials and the solvent are mixed evenly, add the catalyst proline (1.84g, 0.016mol) and lithium chloride (0.68g, 0.016mol) in sequence. After both are completely dissolved, turn on the oil bath Heat to increase the temperature of the reaction solution to 50°C and keep it constant. Regular sampling analysis, GC detection reaction conversion and selectivity, after 4h, GC showed complete conversion of phenylpropionaldehyde (>99%). Add 10% dilute sulfuric acid aqueous solution (15.7g) to the react...
Embodiment 2
[0050] Proline catalyzes the condensation of phenylpropionaldehyde and n-hexanal to synthesize 2-benzyl 2-octenal.
[0051] At room temperature, a 2L three-necked flask equipped with a magnetic stirrer, temperature and reflux condenser was placed in an oil bath, and after the system was replaced 3 times with an oil pump and nitrogen, phenylpropanal (201.3g, 1.5mol ), n-hexanal (150.2g, 1.5mol) and solvent methyl tert-butyl ether (300mL). Turn on the oil bath and stir. After the raw materials and the solvent are mixed evenly, add the catalyst proline (0.86g, 0.0075mol) and lithium chloride (0.64g, 0.015mol) in sequence. After both are completely dissolved, turn on the oil bath Heating to increase the temperature of the reaction solution to 80°C and keep it constant. Regular sampling analysis, GC detection reaction conversion and selectivity, after 2h, GC showed complete conversion of phenylpropanal (>99%). The reaction solution was lowered to 50°C, then 10% dilute sulfuric ac...
Embodiment 3
[0053] Proline catalyzes the condensation of phenylpropionaldehyde and n-hexanal to synthesize 2-benzyl 2-octenal.
[0054] At room temperature, a 3L three-necked flask equipped with a magnetic stirrer, temperature and reflux condenser was placed in an oil bath, and after the system was replaced 3 times with an oil pump and nitrogen, phenylpropanal (214.7g, 1.6mol ), n-hexanal (192.3g, 1.92mol) and solvent methyl tert-butyl ether (800mL). Turn on the oil bath and stir. After the raw materials and the solvent are mixed evenly, add the catalyst proline (9.21g, 0.08mol) and lithium chloride (3.39g, 0.08mol) in sequence. After both are completely dissolved, turn on the oil bath Heat to increase the temperature of the reaction solution to 60°C and keep it constant. Regular sampling analysis, GC detection reaction conversion and selectivity, after 10h, GC showed complete conversion of phenylpropionaldehyde (>99%). Add 10% dilute sulfuric acid aqueous solution (15.7g) to the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com