Method for screening hypoglycemic peptide for inhibiting activity of alpha-amylase
An amylase and blood sugar-lowering technology, which is applied in the field of screening peptides that inhibit α-amylase activity and lower blood sugar, to achieve the effects of reducing interference, improving efficiency, and improving magnetic enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
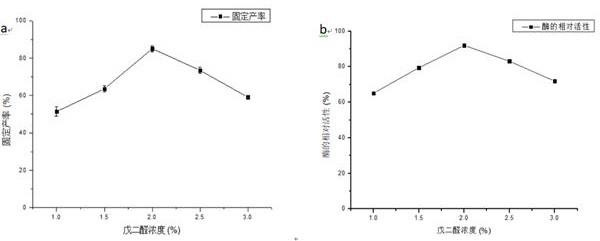
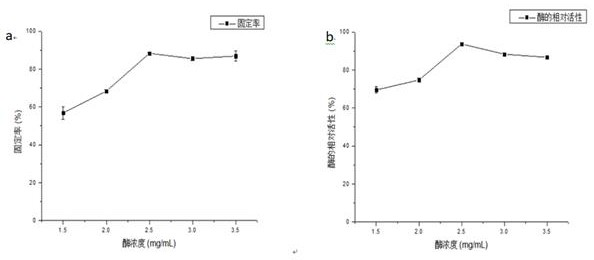
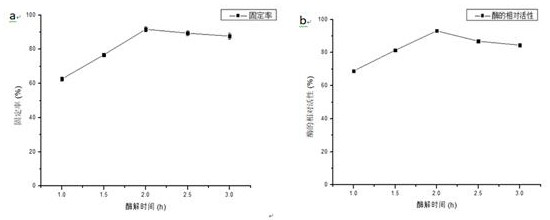
[0031] (1) Press Fe 3 o 4 The ratio of the mass volume ratio of nanoparticles to the mixed solution is 1:10, the Fe 3 o 4 Nanoparticles were suspended in a mixed solution of ethanol and ammonia water (the volume ratio of ethanol and ammonia water was 9:1), sonicated for 30 min, and 300 µL tetramethoxysilane (TMOS) was added to 10 mL of the suspension to shake for 8 h. Repeated washing with ion water 3 times, vacuum drying overnight at 60°C to obtain Fe 3 o 4 @SiO 2 ; 3 o 4 @SiO 2 The infrared spectrum of figure 2As shown in (b), new absorption peaks appear at 802cm−1 and 1096cm−1, which are the bending vibration peaks of Si—OH in the silicon layer and the opposition of Si—O—Si in the silicon layer, respectively. Called stretching peaks, these signals confirm that the silane polymer is bound to the Fe 3 o 4 on the surface of magnetic particles.
[0032] (2) 100 mg of Fe obtained in step (1) 3 o 4 @SiO 2 The particles were suspended in 50mL of isopropanol, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com